Letters in Organic Chemistry ( IF 0.7 ) Pub Date : 2021-02-28 , DOI: 10.2174/1570178617999200713144504 G. Dhananjaya 1 , Akula Raghunadh 1 , P. Mahesh Kumar 1 , S. Pulla Reddy 1 , V. Narayana Murthy 1 , Venkateswara Rao Anna 2 , Manojit Pal 3
|
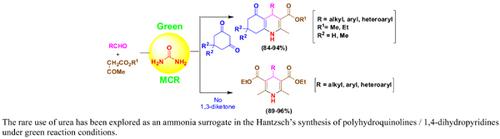
Synthesis of polyhydroquinolines via Hantzsch’s multicomponent reaction (MCR) involves the use of a hygroscopic and moderately toxic ammonium salt as one of the key reactants. In our effort, we have found urea as an effective ammonia surrogate when the MCR was performed in the presence of sulphonic acid-functionalized Wang resin (Wang-OSO3H) as a polymeric and recoverable acidic catalyst under green conditions. Urea is relatively less hygroscopic/toxic than the commonly used ammonium salts used in this MCR. The methodology afforded a range of polyhydroquinolines in good yields. Depending on the nature of reaction conditions employed, the MCR afforded Biginelli product or 1,4-DHPs when the use of 1,3-diketone was omitted.
中文翻译:

在绿色反应条件下尿素作为Hantzsch合成多氢喹啉/ 1,4-二氢吡啶类化合物中的氨替代物
通过Hantzsch多组分反应(MCR)合成聚氢喹啉涉及使用吸湿性和中等毒性的铵盐作为关键反应物之一。在我们的努力中,我们发现,当在绿色条件下,在作为聚合和可回收酸性催化剂的磺酸官能化王树脂(Wang-OSO 3 H)存在下进行MCR时,尿素可作为一种有效的氨替代物。与该MCR中使用的常用铵盐相比,尿素的吸湿性/毒性相对较小。该方法以高收率提供了多种聚氢喹啉。根据所用反应条件的性质,当省去使用1,3-二酮时,MCR提供Biginelli产物或1,4-DHP。











































 京公网安备 11010802027423号
京公网安备 11010802027423号