当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination and Analysis of Solubility of Na3VO4 in Aqueous NaOH Solutions Containing Na2CO3, NaAlO2, or Na2SiO3 at 298.15–353.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-12 , DOI: 10.1021/acs.jced.0c00511 Fancheng Meng 1, 2 , Lei Cao 1, 2 , Huan Yang 1, 2 , Yahui Liu 1, 2 , Lina Wang 1, 2 , Desheng Chen 1, 2 , Hongxin Zhao 1, 2 , Yulan Zhen 1, 2 , Meng Wang 3 , Tao Qi 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-12 , DOI: 10.1021/acs.jced.0c00511 Fancheng Meng 1, 2 , Lei Cao 1, 2 , Huan Yang 1, 2 , Yahui Liu 1, 2 , Lina Wang 1, 2 , Desheng Chen 1, 2 , Hongxin Zhao 1, 2 , Yulan Zhen 1, 2 , Meng Wang 3 , Tao Qi 1, 2
Affiliation
|
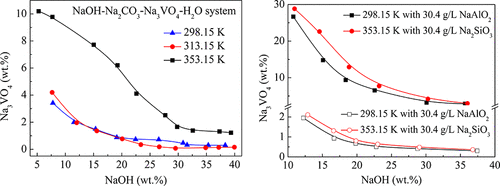
In a new vanadium extracting process, the effective separation of Na3VO4 from the aqueous NaOH solution containing impurities of Na2CO3, NaAlO2, or Na2SiO3 plays a significant role. The solubility data of NaOH-Na3VO4-H2O system and NaOH-Na2CO3-Na3VO4-H2O system at 298.15, 313.15, and 353.15 K were measured. The Na3VO4 solubility in NaOH solutions containing 30.4 g·L–1 NaAlO2 or Na2SiO3 at 313.15 and 353.15 K were also studied for comparison. Na2CO3 has a strong salting-out effect on Na3VO4 with NaOH molality <7 mol·kg–1. Moreover, NaAlO2 and Na2SiO3 have a little inhibitory effect on the Na3VO4 solubility, and the effect of NaAlO2 is more significant. The equilibrium solid phases of sodium orthovanadate in the above systems were identified, which mainly presented Na3VO4·12H2O at NaOH molality <10 mol·kg–1 and Na3VO4 at NaOH molality >10 mol·kg–1. The determined solubility data and equilibrium solid phases are essential in optimizing the operating parameters for Na3VO4 crystallization from actual NaOH solutions. To balance the crystal purity and vanadium recovery, a strategy has been proposed for the effective separation of Na3VO4 by evaporation/cooling crystallization after decreasing the Na2CO3 concentration.
中文翻译:

Na 3 VO 4在含Na 2 CO 3,NaAlO 2或Na 2 SiO 3的NaOH水溶液中在298.15–353.15 K下的溶解度测定和分析
在新的钒提取工艺中,从含有Na 2 CO 3,NaAlO 2或Na 2 SiO 3杂质的NaOH水溶液中有效分离Na 3 VO 4发挥了重要作用。测量了NaOH-Na 3 VO 4 -H 2 O体系和NaOH-Na 2 CO 3 -Na 3 VO 4 -H 2 O体系在298.15、313.15和353.15 K的溶解度数据。Na 3 VO 4在含有30.4 g·L –1 NaAlO的NaOH溶液中的溶解度还研究了在313.15和353.15 K下的2或Na 2 SiO 3进行比较。Na 2 CO 3对Na 3 VO 4的NaOH摩尔浓度<7 mol·kg –1具有很强的盐析作用。此外,NaAlO 2和Na 2 SiO 3对Na 3 VO 4溶解度的抑制作用较小,并且NaAlO 2的作用更为显着。确定了上述体系中原钒酸钠的平衡固相,主要为Na 3 VO 4 ·12H 2。在氢氧化钠摩尔浓度0 <10摩尔公斤· -1和Na 3 VO 4在重量摩尔浓度的NaOH> 10摩尔公斤· -1。确定的溶解度数据和平衡固相对于优化从实际NaOH溶液中Na 3 VO 4结晶的操作参数至关重要。为了平衡晶体纯度和钒的回收率,提出了一种在降低Na 2 CO 3浓度后通过蒸发/冷却结晶有效分离Na 3 VO 4的策略。
更新日期:2021-04-08
中文翻译:

Na 3 VO 4在含Na 2 CO 3,NaAlO 2或Na 2 SiO 3的NaOH水溶液中在298.15–353.15 K下的溶解度测定和分析
在新的钒提取工艺中,从含有Na 2 CO 3,NaAlO 2或Na 2 SiO 3杂质的NaOH水溶液中有效分离Na 3 VO 4发挥了重要作用。测量了NaOH-Na 3 VO 4 -H 2 O体系和NaOH-Na 2 CO 3 -Na 3 VO 4 -H 2 O体系在298.15、313.15和353.15 K的溶解度数据。Na 3 VO 4在含有30.4 g·L –1 NaAlO的NaOH溶液中的溶解度还研究了在313.15和353.15 K下的2或Na 2 SiO 3进行比较。Na 2 CO 3对Na 3 VO 4的NaOH摩尔浓度<7 mol·kg –1具有很强的盐析作用。此外,NaAlO 2和Na 2 SiO 3对Na 3 VO 4溶解度的抑制作用较小,并且NaAlO 2的作用更为显着。确定了上述体系中原钒酸钠的平衡固相,主要为Na 3 VO 4 ·12H 2。在氢氧化钠摩尔浓度0 <10摩尔公斤· -1和Na 3 VO 4在重量摩尔浓度的NaOH> 10摩尔公斤· -1。确定的溶解度数据和平衡固相对于优化从实际NaOH溶液中Na 3 VO 4结晶的操作参数至关重要。为了平衡晶体纯度和钒的回收率,提出了一种在降低Na 2 CO 3浓度后通过蒸发/冷却结晶有效分离Na 3 VO 4的策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号