当前位置:
X-MOL 学术
›
J. Mol. Recognit.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In silico prediction, molecular docking and binding studies of acetaminophen and dexamethasone to Enterococcus faecalis diaminopimelate epimerase
Journal of Molecular Recognition ( IF 2.7 ) Pub Date : 2021-03-14 , DOI: 10.1002/jmr.2894 Harpreet Singh 1 , Satyajeet Das 1 , Jyoti Yadav 1 , Vijay Kumar Srivastava 1 , Anupam Jyoti 1 , Sanket Kaushik 1
Journal of Molecular Recognition ( IF 2.7 ) Pub Date : 2021-03-14 , DOI: 10.1002/jmr.2894 Harpreet Singh 1 , Satyajeet Das 1 , Jyoti Yadav 1 , Vijay Kumar Srivastava 1 , Anupam Jyoti 1 , Sanket Kaushik 1
Affiliation
|
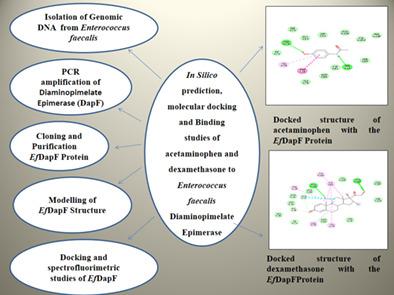
Enterococcus faecalis (E. faecalis) is a Gram-positive coccoid, non-sporulating, facultative anaerobic, multidrug resistance bacterium responsible for almost 65% to 80% of all enterococcal nosocomial infections. It usually causes infective endocarditis, urinary tract and surgical wound infections. The increase in E. faecalis resistance to conventionally available antibiotic has rekindled intense interest in developing useful antibacterial drugs. In E. faecalis, diaminopimelate epimerase (DapF) is involved in the lysine biosynthetic pathway. The product of this pathway is precursors of peptidoglycan synthesis, which is a component of bacterial cell wall. Also, because mammals lack this enzyme, consequently E. faecalis diaminopimelate epimerase (EfDapF) represents a potential target for developing novel class of antibiotics. In this regard, we have successfully cloned, overexpressed the gene encoding DapF in BL-21(DE3) and purified with Ni-NTA Agarose resin. In addition to this, binding studies were performed using fluorescence spectroscopy in order to confirm the bindings of the identified lead compounds (acetaminophen and dexamethasone) with EfDapF. Docking studies revealed that acetaminophen found to make hydrogen bonds with Asn72 and Asn13 while dexamethasone interacted by forming hydrogen bonds with Asn205 and Glu223. Thus, biochemical studies indicated acetaminophen and dexamethasone, as potential inhibitors of EfDapF and eventually can reduce the catalytic activity of EfDapF.
中文翻译:

对乙酰氨基酚和地塞米松与粪肠球菌二氨基庚二酸差向异构酶的计算机预测、分子对接和结合研究
粪肠球菌( E. faecalis )是革兰氏阳性球菌、非孢子形成、兼性厌氧、多药耐药细菌,导致几乎 65% 至 80% 的所有肠球菌医院感染。它通常引起感染性心内膜炎、泌尿道和手术伤口感染。E的增加。粪肠球菌对常规可用抗生素的耐药性重新激发了人们对开发有用的抗菌药物的强烈兴趣。在E中。粪便, 二氨基庚二酸差向异构酶 (DapF) 参与赖氨酸生物合成途径。该途径的产物是肽聚糖合成的前体,肽聚糖是细菌细胞壁的组成部分。此外,由于哺乳动物缺乏这种酶,因此E。粪肠球菌二氨基庚二酸差向异构酶 ( Ef DapF) 代表了开发新型抗生素的潜在靶点。在这方面,我们已成功克隆、过表达BL-21(DE3)中编码DapF的基因,并用Ni-NTA琼脂糖树脂纯化。除此之外,使用荧光光谱进行结合研究以确认已鉴定的先导化合物(对乙酰氨基酚和地塞米松)与Ef的结合磷酸二铵。对接研究表明,对乙酰氨基酚发现与 Asn72 和 Asn13 形成氢键,而地塞米松通过与 Asn205 和 Glu223 形成氢键相互作用。因此,生化研究表明对乙酰氨基酚和地塞米松是Ef DapF的潜在抑制剂,并最终会降低Ef DapF 的催化活性。
更新日期:2021-03-14
中文翻译:

对乙酰氨基酚和地塞米松与粪肠球菌二氨基庚二酸差向异构酶的计算机预测、分子对接和结合研究
粪肠球菌( E. faecalis )是革兰氏阳性球菌、非孢子形成、兼性厌氧、多药耐药细菌,导致几乎 65% 至 80% 的所有肠球菌医院感染。它通常引起感染性心内膜炎、泌尿道和手术伤口感染。E的增加。粪肠球菌对常规可用抗生素的耐药性重新激发了人们对开发有用的抗菌药物的强烈兴趣。在E中。粪便, 二氨基庚二酸差向异构酶 (DapF) 参与赖氨酸生物合成途径。该途径的产物是肽聚糖合成的前体,肽聚糖是细菌细胞壁的组成部分。此外,由于哺乳动物缺乏这种酶,因此E。粪肠球菌二氨基庚二酸差向异构酶 ( Ef DapF) 代表了开发新型抗生素的潜在靶点。在这方面,我们已成功克隆、过表达BL-21(DE3)中编码DapF的基因,并用Ni-NTA琼脂糖树脂纯化。除此之外,使用荧光光谱进行结合研究以确认已鉴定的先导化合物(对乙酰氨基酚和地塞米松)与Ef的结合磷酸二铵。对接研究表明,对乙酰氨基酚发现与 Asn72 和 Asn13 形成氢键,而地塞米松通过与 Asn205 和 Glu223 形成氢键相互作用。因此,生化研究表明对乙酰氨基酚和地塞米松是Ef DapF的潜在抑制剂,并最终会降低Ef DapF 的催化活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号