当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ideal-Gas Thermochemical Properties for Alkanolamine and Related Species Involved in Carbon-Capture Applications
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-12 , DOI: 10.1021/acs.jced.0c00742 Nayyereh Hatefi 1 , William R. Smith 2, 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-12 , DOI: 10.1021/acs.jced.0c00742 Nayyereh Hatefi 1 , William R. Smith 2, 3
Affiliation
|
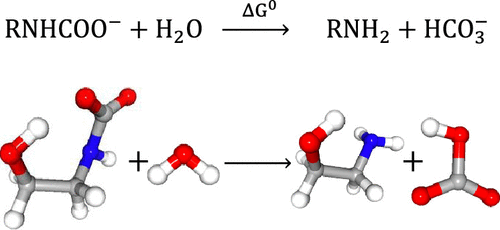
We calculated ideal-gas thermochemical properties (molar enthalpy, entropy, Gibbs energy, and heat capacity, Cp) for 30 primary and 12 secondary alkanolamines and their neutral, protonated, and carbamate forms, and for 7 tertiary alkanolamines and their protonated forms. The data, provided in a spreadsheet format in the Supporting Information document, are useful for the construction of both macroscopic and molecular-based thermodynamic models of CO2-capture processes involving the indicated species. The calculations were implemented using a combination of Spartan18 and Gaussian16 electronic structure software using two typical high-order methods, G4 and G3B3, and for comparison, we also calculated results from the commonly used and less computationally expensive B3LYP/aug-cc-pVTZ method. Our calculation methodology is validated by comparing results for a set of molecules for which literature data are available. The thermochemical data for each species over the temperature range 200–1500 K is given as a function of temperature in the form of NASA seven-term polynomial expressions, permitting the properties to be calculated over this temperature range. The accuracy of the G3B3 and G4 results is estimated to be 6 kJ·mol–1 for the species formation enthalpies, and the B3LYP/aug-cc-pVTZ results are generally of inferior quality.
中文翻译:

烷醇胺及相关碳捕获应用相关物种的理想气体热化学性质
我们计算了30种伯和12种仲烷醇胺及其中性,质子化和氨基甲酸酯形式以及7种叔烷醇胺及其质子化形式的理想气体热化学性质(摩尔焓,熵,吉布斯能量和热容C p)。支持信息文档中以电子表格格式提供的数据对于构建宏观和基于分子的CO 2热力学模型都非常有用-捕获涉及所示物种的过程。使用Spartan18和Gaussian16电子结构软件结合使用两种典型的高阶方法G4和G3B3来进行计算,为了进行比较,我们还从常用且计算成本较低的B3LYP / aug-cc-pVTZ方法计算了结果。通过比较可获得文献数据的一组分子的结果,我们的计算方法得到了验证。在200–1500 K的温度范围内,每个物种的热化学数据以温度的函数形式以NASA七项多项式的形式给出,从而可以在该温度范围内计算特性。G3B3和G4结果的准确性估计为6 kJ·mol –1 对于物种形成焓,B3LYP / aug-cc-pVTZ结果通常质量较差。
更新日期:2021-04-08
中文翻译:

烷醇胺及相关碳捕获应用相关物种的理想气体热化学性质
我们计算了30种伯和12种仲烷醇胺及其中性,质子化和氨基甲酸酯形式以及7种叔烷醇胺及其质子化形式的理想气体热化学性质(摩尔焓,熵,吉布斯能量和热容C p)。支持信息文档中以电子表格格式提供的数据对于构建宏观和基于分子的CO 2热力学模型都非常有用-捕获涉及所示物种的过程。使用Spartan18和Gaussian16电子结构软件结合使用两种典型的高阶方法G4和G3B3来进行计算,为了进行比较,我们还从常用且计算成本较低的B3LYP / aug-cc-pVTZ方法计算了结果。通过比较可获得文献数据的一组分子的结果,我们的计算方法得到了验证。在200–1500 K的温度范围内,每个物种的热化学数据以温度的函数形式以NASA七项多项式的形式给出,从而可以在该温度范围内计算特性。G3B3和G4结果的准确性估计为6 kJ·mol –1 对于物种形成焓,B3LYP / aug-cc-pVTZ结果通常质量较差。











































 京公网安备 11010802027423号
京公网安备 11010802027423号