Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2021-03-10 , DOI: 10.1016/j.jwpe.2021.102008 Sabah Mohamed Abdelbasir , Aya Mostafa El-Shewaikh , Said Moawad El-Sheikh , Omnia Ibrahim Ali
|
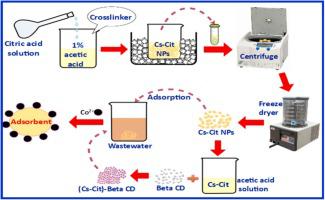
Herein, novel and efficient nanocomposites of modified chitosan (Cs) with citric acid (Cit.) and beta-cyclodextrin (Beta CD) were manufactured and explored, first-ever, to remove cobalt ions (Co) pollutant from wastewater. The prepared adsorbents, Cs-Cit and (Cs-Cit)-Beta CD were scrutinized via X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), energy dispersive X-ray (EDX), scanning electron microscopy (SEM), and transmission electron microscope (TEM). The uptake capacities of Co2+ using different samples were studied. Factors influencing the sorption of Co2+; for example, time, pH, dosage of adsorbent, preliminary ions concentration, and temperature were studied. The maximum adsorption capacities of Co2+ using raw Cs, Cs-Cit, and (Cs-Cit)-Beta CD were found to be 6.90, 18.50, and 43.95 mg.g−1, respectively. Kinetics pursued a pseudo-second-order paradigm and thermodynamic studies suggested that Co2+ sorption on the modified chitosan adsorbents was exothermic compared to the endothermic nature of the raw Cs adsorption. Adsorption–desorption experiments affirmed the renewability and competitiveness of the prepared materials as adsorbents for Co2+ removal. As well, applications of these adsorbents were investigated for cobalt ions elimination from real water samples (tap, Nile, industrial water samples) confirming they are considered virtuous candidates for wastewater treatment.
中文翻译:

新型改性壳聚糖纳米复合材料用于去除工业废水中的Co(II)离子
在这里,有史以来首次制造并探索了新型,高效的改性壳聚糖(Cs)与柠檬酸(Cit。)和β-环糊精(Beta CD)的纳米复合材料,以去除废水中的钴离子(Co)污染物。通过X射线衍射(XRD),傅立叶变换红外光谱(FTIR),能量色散X射线(EDX),扫描电子显微镜(SEM)对制备的吸附剂Cs-Cit和(Cs-Cit)-Beta CD进行仔细检查,以及透射电子显微镜(TEM)。研究了不同样品对Co 2+的吸收能力。影响有限的吸附因素2+ ; 例如,研究了时间,pH,吸附剂的用量,初步离子浓度和温度。Co 2+的最大吸附量使用原始Cs,Cs-Cit和(Cs-Cit)-Beta CD的结果分别为6.90、18.50和43.95 mg.g -1。动力学遵循伪二级范式,热力学研究表明,与原始Cs吸附的吸热性质相比,改性壳聚糖吸附剂对Co 2+的吸附是放热的。吸附-解吸实验证实了所制备材料作为去除Co 2+的吸附剂的可再生性和竞争力。同样,还对这些吸附剂的应用进行了研究,以从真实水样(自来水,尼罗河,工业水样)中消除钴离子,从而确认了它们被认为是废水处理的有益候选物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号