当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The System CaCl2–H2O: Thermodynamic Modeling and Flow Calorimetry Experiments at Elevated Temperatures and Pressures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-10 , DOI: 10.1021/acs.jced.0c00951 Chad M. Able 1, 2 , Jason P. Trembly 1, 2, 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-10 , DOI: 10.1021/acs.jced.0c00951 Chad M. Able 1, 2 , Jason P. Trembly 1, 2, 3
Affiliation
|
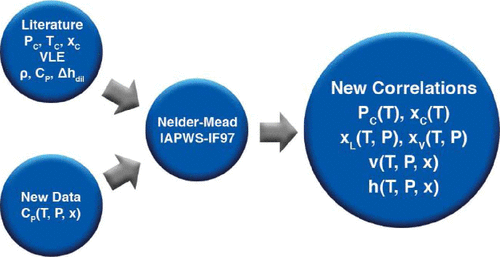
Produced water from oil and natural gas production is challenging to treat due to its high salinity. Such brines are often a mixture of various mono- and divalent-cation salts, and elevated pressure methods near water’s critical point to reclaim reusable water from the brines for reuse are of growing interest. Modeling efforts have traditionally focused on NaCl–H2O systems, which potentially introduces considerable calculation error when simulating multi-component salt solutions. This study considers the thermodynamic characteristics of the CaCl2–H2O system to aid in this effort. A series of correlations are presented for the critical pressure line and composition, vapor–liquid equilibria around the critical line, and specific volume and enthalpy for high-temperature and pressure CaCl2–H2O systems. In addition, heat capacity data for CaCl2–H2O are presented near the critical line to assist in the determination of the specific enthalpy correlation, with mass fractions from 0.1 to 0.2, pressure from 23 to 26 MPa, and temperatures from 582 to 625 K.
中文翻译:

系统CaCl 2 –H 2 O:在升高的温度和压力下的热力学建模和流热法实验
石油和天然气生产中产生的水由于其高盐度而难以处理。这样的盐水通常是各种一价和二价阳离子盐的混合物,并且在水的临界点附近的高压方法中,从盐水中回收可再利用的水以进行再利用越来越引起人们的关注。传统上,建模工作集中在NaCl–H 2 O系统上,这在模拟多组分盐溶液时可能会引入相当大的计算误差。这项研究考虑了CaCl 2 -H 2的热力学特性O系统可协助此工作。对于临界压力线和组成,临界线周围的气液平衡以及高温和高压CaCl 2 -H 2 O系统的比容和焓,提出了一系列相关性。此外,在临界线附近还提供了CaCl 2 -H 2 O的热容数据,以帮助确定比焓相关性,质量分数从0.1到0.2,压力从23到26 MPa,温度从582到120 MPa。 625千
更新日期:2021-04-08
中文翻译:

系统CaCl 2 –H 2 O:在升高的温度和压力下的热力学建模和流热法实验
石油和天然气生产中产生的水由于其高盐度而难以处理。这样的盐水通常是各种一价和二价阳离子盐的混合物,并且在水的临界点附近的高压方法中,从盐水中回收可再利用的水以进行再利用越来越引起人们的关注。传统上,建模工作集中在NaCl–H 2 O系统上,这在模拟多组分盐溶液时可能会引入相当大的计算误差。这项研究考虑了CaCl 2 -H 2的热力学特性O系统可协助此工作。对于临界压力线和组成,临界线周围的气液平衡以及高温和高压CaCl 2 -H 2 O系统的比容和焓,提出了一系列相关性。此外,在临界线附近还提供了CaCl 2 -H 2 O的热容数据,以帮助确定比焓相关性,质量分数从0.1到0.2,压力从23到26 MPa,温度从582到120 MPa。 625千









































 京公网安备 11010802027423号
京公网安备 11010802027423号