当前位置:
X-MOL 学术
›
Can. J. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient extraction and theoretical insights for separating o-, m-, and p-cresol from model coal tar by an ionic liquid [Emim][DCA]
The Canadian Journal of Chemical Engineering ( IF 1.6 ) Pub Date : 2021-03-09 , DOI: 10.1002/cjce.24104 Ao Li 1 , Chen Zhu 1 , Lianzheng Zhang 1 , Yixin Ma 1 , Dongmei Xu 1 , Jun Gao 1 , Yinglong Wang 2
The Canadian Journal of Chemical Engineering ( IF 1.6 ) Pub Date : 2021-03-09 , DOI: 10.1002/cjce.24104 Ao Li 1 , Chen Zhu 1 , Lianzheng Zhang 1 , Yixin Ma 1 , Dongmei Xu 1 , Jun Gao 1 , Yinglong Wang 2
Affiliation
|
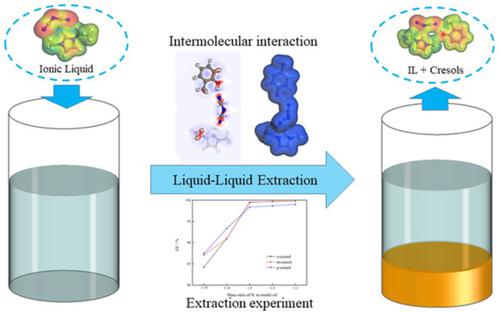
Cresols are valuable phenolic chemicals which are mainly separated from coal tar. The separation of cresols using sustainable technology is of importance for comprehensive utilization of coal tar. In this work, for extracting o-, m-, and p-cresol from the model coal tar, an ionic liquid 1-ethyl-3-methylimidazolium dicyanamide ([Emim][DCA]) was prepared. The influence of ionic liquid dosage, extraction time, and temperature on separating o-, m-, and p-cresol was explored. The extraction efficiency was determined from the experimental results to assess the extraction ability of [Emim][DCA]. With the optimal conditions, o-, m-, and p-cresol were extracted efficiently from the model coal tar and the extraction efficiencies of o-cresol, m-cresol, and p-cresol were up to 99.50%, 99.65%, 98.38%, respectively. In the meantime, the extraction mechanism was determined by calculation of σ-profile, interaction energy, and electron density. The results showed that [Emim][DCA] and cresols formed the complexes by hydrogen bond. The formation of hydrogen bond was verified by the FTIR spectra, which is helpful for designing the related ionic liquids for recovering the phenolic compounds from coal tar. In addition, the recyclability of the [Emim][DCA] was explored.
中文翻译:

离子液体从模型煤焦油中分离邻甲酚、间甲酚和对甲酚的有效萃取和理论见解 [Emim][DCA]
甲酚是有价值的酚类化学品,主要从煤焦油中分离出来。采用可持续技术分离甲酚对煤焦油的综合利用具有重要意义。在这项工作中,为了从模型煤焦油中提取邻、间和对甲酚,制备了离子液体 1-乙基-3-甲基咪唑鎓双氰胺 ([Emim][DCA])。探讨了离子液体用量、萃取时间和温度对分离邻甲酚、间甲酚和对甲酚的影响。根据实验结果确定提取效率,以评估 [Emim][DCA] 的提取能力。在最优条件下,o -、m - 和从模型煤焦油中高效提取对甲酚,邻甲酚、间甲酚和对甲酚的提取率分别高达99.50%、99.65%、98.38%。同时,通过计算σ-分布、相互作用能和电子密度来确定萃取机理。结果表明[Emim][DCA]与甲酚通过氢键形成配合物。FTIR光谱验证了氢键的形成,有助于设计相关离子液体从煤焦油中回收酚类化合物。此外,还探讨了 [Emim][DCA] 的可回收性。
更新日期:2021-03-09
中文翻译:

离子液体从模型煤焦油中分离邻甲酚、间甲酚和对甲酚的有效萃取和理论见解 [Emim][DCA]
甲酚是有价值的酚类化学品,主要从煤焦油中分离出来。采用可持续技术分离甲酚对煤焦油的综合利用具有重要意义。在这项工作中,为了从模型煤焦油中提取邻、间和对甲酚,制备了离子液体 1-乙基-3-甲基咪唑鎓双氰胺 ([Emim][DCA])。探讨了离子液体用量、萃取时间和温度对分离邻甲酚、间甲酚和对甲酚的影响。根据实验结果确定提取效率,以评估 [Emim][DCA] 的提取能力。在最优条件下,o -、m - 和从模型煤焦油中高效提取对甲酚,邻甲酚、间甲酚和对甲酚的提取率分别高达99.50%、99.65%、98.38%。同时,通过计算σ-分布、相互作用能和电子密度来确定萃取机理。结果表明[Emim][DCA]与甲酚通过氢键形成配合物。FTIR光谱验证了氢键的形成,有助于设计相关离子液体从煤焦油中回收酚类化合物。此外,还探讨了 [Emim][DCA] 的可回收性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号