Forensic Chemistry ( IF 2.7 ) Pub Date : 2021-03-10 , DOI: 10.1016/j.forc.2021.100330 Mirjam de Bruin-Hoegée , Djarah Kleiweg , Daan Noort , Arian C. van Asten
|
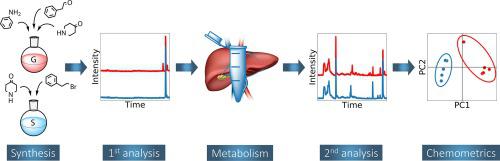
Chemical attribution typically aims to establish a link between material found at a crime scene and a person, location or other evidence. In the field of illicit drugs, chemical attribution signatures are usually impurity profiles. Extending these to metabolized samples would create new possibilities in forensic investigations. The present study explores the effect of human metabolism on the impurity profile of fentanyl, as representative of synthetic opioids. Two different methods (Gupta and Siegfried) were used to synthesize fentanyl, after which the samples were incubated with liver microsomes to mimic human metabolism. The impurity profiles have been characterized with gas chromatography-mass spectrometry (GC-MS), gas chromatography with flame ionization detector (GC-FID), liquid chromatography quadrupole-time of flight mass spectrometry (LC-Q-TOF-MS) and liquid chromatography orbitrap mass spectrometry (LC-Orbitrap-MS). It was found that GC-FID and LC-Orbitrap-MS can both be used to discriminate between the Gupta and Siegfried synthesis method. This holds both for the analyses performed before and after metabolism. In addition, principal component analysis (PCA) identified acetyl fentanyl as the most important marker compound. Associated detection limits are in the range of concentrations expected in case work. While acetyl fentanyl is not stable during metabolism, its discriminating potential is transferred to its metabolic product acetyl norfentanyl. In addition, the stable impurities phenylacetamide and 1-phenylethylpiperidin-4-ol were found to be significant classifiers. To implement the results in a forensic framework, linear discriminant analysis (LDA) was applied and used to establish likelihood ratios. To our knowledge, the present work demonstrates for the first time the possibility of chemical attribution of drugs through the analysis of metabolic trace levels in biological samples.
中文翻译:

芬太尼的化学归因:人类新陈代谢的影响
化学归因通常旨在在犯罪现场发现的材料与人,地点或其他证据之间建立联系。在非法药物领域,化学属性特征通常是杂质概况。将其扩展到代谢样品将为法医研究创造新的可能性。本研究探讨了人类代谢对作为合成阿片类药物代表的芬太尼杂质谱的影响。两种不同的方法(古普塔(Gupta)和齐格弗里德(Siegfried))被用于合成芬太尼,然后将样品与肝微粒体一起温育以模拟人的新陈代谢。使用气相色谱-质谱(GC-MS),火焰离子化检测器(GC-FID),液相色谱四极杆飞行时间质谱(LC-Q-TOF-MS)和液相色谱轨道阱质谱(LC-Orbitrap-MS)。发现GC-FID和LC-Orbitrap-MS均可用于区分古普塔和齐格弗里德的合成方法。这适用于新陈代谢之前和之后进行的分析。此外,主成分分析(PCA)将乙酰芬太尼确定为最重要的标记化合物。相关的检出限在案例工作中预期的浓度范围内。尽管乙酰芬太尼在代谢过程中不稳定,但其识别潜力却转移到了其代谢产物乙酰降芬太尼中。另外,发现稳定的杂质苯乙酰胺和1-苯基乙基哌啶-4-醇是重要的分类器。为了在法医框架中实施结果,应用了线性判别分析(LDA)并用于建立似然比。据我们所知,本工作首次通过分析生物样品中的微量代谢水平证明了药物化学归因的可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号