FlatChem ( IF 5.9 ) Pub Date : 2021-03-10 , DOI: 10.1016/j.flatc.2021.100238 Ahmed El Hadki , Kubra Ulucan-Altuntas , Hamza El Hadki , Cem Bulent Ustundag , Oum Keltoum Kabbaj , Abdelmalek Dahchour , Najia Komiha , Abdallah Zrineh , Eyup Debik
|
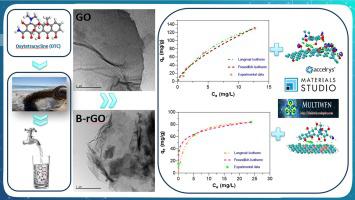
Graphene-based nanomaterials have demonstrated great potential in the field of environmental applications, especially on water treatment processes. Accordingly, herein, in order to be used as adsorbent in the removal of oxytetracycline (OTC), graphene oxide (GO) and boron doped reduced graphene oxide (B-rGO) was investigated. GO was obtained through the oxidation/exfoliation process using the modified Hummers’ Method and further etched by a thermal annealing approach to obtain B-rGO, utilizing boric acid as boron source for the study. FT-IR, TEM and XRD were used to characterize the morphology properties of GO and B-rGO and confirm the success of these synthesis. To evaluate the degradation potential of OTC by GO and B-rGO, pH of the sample, GO and B-rGO concentration, initial OTC concentration, reaction time and temperature has been selected as effective parameters. Based on the obtained experimental results GO and B-rGO were found to possess favorable adsorption efficiencies reaching 86% and 100% for GO and B-rGO, respectively, rapidly uptake rate with up to 85% of total removal occurring within the initial 10 min. In addition, it is noteworthy that OTC removal from solution was strongly dependent on pH but independent of temperature. The classical isotherm and kinetic adsorption models suggested that the process perfectly conformed to Freundlich and Pseudo-second-order model (R2 ≥ 0.95). Furthermore, density functional theory (DFT) simulation performed at the B3PW91 level of theory as well as a topological analysis were introduced to elucidate theoretically the interfacial interaction at the molecular-scale associated with OTC sorption on both adsorbents.
中文翻译:

氧化石墨烯和硼掺杂的还原氧化石墨烯去除土霉素的研究:组合的密度泛函理论,分子动力学模拟和实验研究
石墨烯基纳米材料在环境应用领域,特别是在水处理过程中已显示出巨大的潜力。因此,在本文中,为了在氧化四环素(OTC)的去除中用作吸附剂,研究了氧化石墨烯(GO)和掺硼还原的氧化石墨烯(B-rGO)。通过使用改进的Hummers方法通过氧化/剥离工艺获得GO,然后使用硼酸作为硼源,通过热退火方法进一步蚀刻以获得B-rGO。FT-IR,TEM和XRD表征了GO和B-rGO的形貌特征,并证实了这些合成方法的成功。为了评估GO和B-rGO对OTC的降解潜力,样品的pH值,GO和B-rGO浓度,初始OTC浓度,选择反应时间和温度作为有效参数。根据获得的实验结果,发现GO和B-rGO对GO和B-rGO的吸附效率分别达到86%和100%,快速吸收速率,最初10分钟内高达85%的总去除率发生。 。此外,值得注意的是,从溶液中去除OTC很大程度上取决于pH值,但与温度无关。经典的等温线和动力学吸附模型表明该过程完全符合Freundlich和伪二阶模型(R 快速吸收速率,在最初的10分钟之内发生的总清除量高达85%。此外,值得注意的是,从溶液中去除OTC很大程度上取决于pH值,但与温度无关。经典的等温线和动力学吸附模型表明该过程完全符合Freundlich和伪二阶模型(R 快速吸收速率,在最初的10分钟之内发生的总清除量高达85%。此外,值得注意的是,从溶液中去除OTC很大程度上取决于pH值,但与温度无关。经典的等温线和动力学吸附模型表明该过程完全符合Freundlich和伪二阶模型(R2 ≥0.95)。此外,引入了在B3PW91的理论水平上进行的密度泛函理论(DFT)模拟以及拓扑分析,以从理论上阐明与两种吸附剂上的OTC吸附相关的分子尺度上的界面相互作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号