Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-03-10 , DOI: 10.1016/j.jmgm.2021.107897 Jonathan Maiangwa 1 , Siti Hajar Hamdan 2 , Mohd Shukuri Mohamad Ali 2 , Abu Bakar Salleh 3 , Raja Noor Zaliha Raja Abd Rahman 4 , Fairolniza Mohd Shariff 5 , Thean Chor Leow 6
|
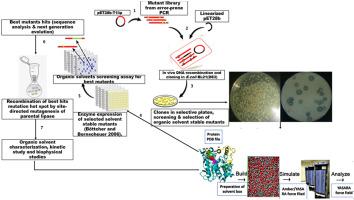
Critical to the applications of proteins in non-aqueous enzymatic processes is their structural dynamics in relation to solvent polarity. A pool of mutants derived from Geobacillus zalihae T1 lipase was screened in organic solvents (methanol, ethanol, propanol, butanol and pentanol) resulting in the selection of six mutants at initial screening (A83D/K251E, R21C, G35D/S195 N, K84R/R103C/M121I/T272 M and R106H/G327S). Site-directed mutagenesis further yielded quadruple mutants A83D/M121I/K251E/G327S and A83D/M121I/S195 N/T272 M, both of which had improved activity after incubation in methanol. The km and kcat values of these mutants vary marginally with the wild-type enzyme in the methanol/substrate mixture. Thermally induced unfolding of mutants was accompanied with some loss of secondary structure content. The root mean square deviations (RMSD) and B-factors revealed that changes in the structural organization are intertwined with an interplay of the protein backbone with organic solvents. Spatially exposed charged residues showed correlations between the solvation dynamics of the methanol solvent and the hydrophobicity of the residues. The short distances of the radial distribution function provided the required distances for hydrogen bond formation and hydrophobic interactions. These dynamic changes demonstrate newly formed structural interactions could be targeted and incorporated experimentally on the basis of solvent mobility and mutant residues.
中文翻译:

增强扎里海芽孢杆菌T1脂肪酶在有机溶剂中的稳定性,并深入了解其变体的结构稳定性
对于蛋白质在非水酶过程中的应用至关重要的是它们相对于溶剂极性的结构动力学。在有机溶剂(甲醇,乙醇,丙醇,丁醇和戊醇)中筛选了一组来自扎利哈芽孢杆菌T1脂肪酶的突变体,从而在初始筛选时选择了六个突变体(A83D / K251E,R21C,G35D / S195 N,K84R / R103C / M121I / T272 M和R106H / G327S)。定点诱变进一步产生了四个突变体A83D / M121I / K251E / G327S和A83D / M121I / S195 N / T272 M,它们在甲醇中孵育后均具有改善的活性。的ķ米和ķ猫这些突变体的值随甲醇/底物混合物中的野生型酶而略有变化。热诱导的突变体的展开伴随着二级结构含量的一些损失。均方根偏差(RMSD)和B因子表明,结构组织的变化与蛋白质主链与有机溶剂的相互作用交织在一起。空间暴露的带电残基显示出甲醇溶剂的溶剂化动力学与残基的疏水性之间的相关性。径向分布函数的短距离提供了氢键形成和疏水相互作用所需的距离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号