当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Enantiopure Benzo Fused Cyclic Sulfoximines Through Stereoselective [3+2] Cycloaddition between N‐tert‐Butanesulfinyl [(2‐Pyridyl)sulfonyl]‐difluoromethyl Ketimines and Arynes
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2021-03-09 , DOI: 10.1002/hlca.202100019 Jian Rong 1 , Chuanfa Ni 2 , Yucheng Gu 3 , Jinbo Hu 4
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2021-03-09 , DOI: 10.1002/hlca.202100019 Jian Rong 1 , Chuanfa Ni 2 , Yucheng Gu 3 , Jinbo Hu 4
Affiliation
|
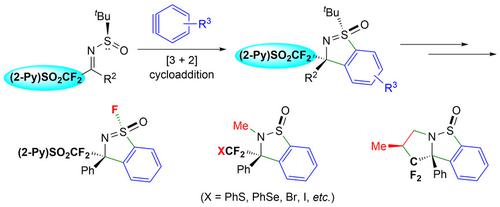
The previously developed stereoselective [3+2] cycloaddition between N‐tert‐butanesulfinyl ketimines and arynes has been extended to the synthesis of enantiopure [(2‐pyridyl)sulfonyl]difluoromethylated cyclic sulfoximines. The use of 2‐PySO2CF2 as the facilitating group offers new opportunities for the elaboration of the [3+2] cycloaddition products by virtue of the diverse relativity of 2‐pyridyl sulfones.
中文翻译:

N-叔丁烷亚磺酰基[(2-吡啶基)磺酰基]-二氟甲基酮亚胺和Arynes之间的立体选择性[3 + 2]环加成反应合成对映体纯的苯并稠合的环状亚砜
之间的先前开发的立体选择[3 + 2]环加成Ñ -叔-butanesulfinyl酮亚胺和arynes一直延伸到对映体纯的合成[(2-吡啶基)磺酰基] difluoromethylated环状亚磺酰亚胺。由于2-吡啶基砜的相对性不同,使用2-PySO 2 CF 2作为促进基团为[3 + 2]环加成产物的精制提供了新的机会。
更新日期:2021-04-14
中文翻译:

N-叔丁烷亚磺酰基[(2-吡啶基)磺酰基]-二氟甲基酮亚胺和Arynes之间的立体选择性[3 + 2]环加成反应合成对映体纯的苯并稠合的环状亚砜
之间的先前开发的立体选择[3 + 2]环加成Ñ -叔-butanesulfinyl酮亚胺和arynes一直延伸到对映体纯的合成[(2-吡啶基)磺酰基] difluoromethylated环状亚磺酰亚胺。由于2-吡啶基砜的相对性不同,使用2-PySO 2 CF 2作为促进基团为[3 + 2]环加成产物的精制提供了新的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号