Journal of Structural Biology ( IF 3 ) Pub Date : 2021-03-09 , DOI: 10.1016/j.jsb.2021.107715 Éverton Dias D'Andréa 1 , Joren Sebastian Retel 2 , Anne Diehl 2 , Peter Schmieder 2 , Hartmut Oschkinat 3 , José Ricardo Pires 1
|
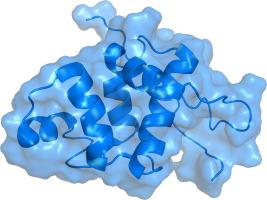
The 106-residue protein Q4DY78 (UniProt accession number) from Trypanosoma cruzi is highly conserved in the related kinetoplastid pathogens Trypanosoma brucei and Leishmania major. Given the essentiality of its orthologue in T. brucei, the high sequence conservation with other trypanosomatid proteins, and the low sequence similarity with mammalian proteins, Q4DY78 is an attractive protein for structural characterization. Here, we solved the structure of Q4DY78 by solution NMR and evaluated its backbone dynamics. Q4DY78 is composed of five α -helices and a small, two-stranded antiparallel β-sheet. The backbone RMSD is 0.22 ± 0.05 Å for the representative ensemble of the 20 lowest-energy structures. Q4DY78 is overall rigid, except for N-terminal residues (V8 to I10), residues at loop 4 (K57 to G65) and residues at the C-terminus (F89 to F112). Q4DY78 has a short motif FPCAP that could potentially mediate interactions with the host cytoskeleton via interaction with EVH1 (Drosophila Enabled (Ena)/Vasodilator-stimulated phosphoprotein (VASP) homology 1) domains. Albeit Q4DY78 lacks calcium-binding motifs, its fold resembles that of eukaryotic calcium-binding proteins such as calcitracin, calmodulin, and polcacin Bet V4. We characterized this novel protein with a calcium binding fold without the capacity to bind calcium.
中文翻译:

Q4DY78的核磁共振结构和动力学,一种来自克氏锥虫的保守动质体特异性蛋白
来自克氏锥虫的 106 个残基蛋白 Q4DY78(UniProt 登录号)在相关的动质体病原体布氏锥虫和大利什曼原虫中高度保守。鉴于其直系同源物在T. brucei中的重要性,与其他锥虫蛋白的高序列保守性,以及与哺乳动物蛋白的低序列相似性,Q4DY78 是用于结构表征的有吸引力的蛋白质。在这里,我们通过溶液 NMR 解决了 Q4DY78 的结构并评估了其骨架动力学。Q4DY78 由五个 α 螺旋和一个小的两链反平行 β 折叠组成。对于 20 个最低能量结构的代表性集合,主干 RMSD 为 0.22 ± 0.05 Å。Q4DY78 总体上是刚性的,除了 N 端残基(V 8到 I 10)、环 4 处的残基(K 57到 G 65)和 C 端的残基(F 89到 F 112)。Q4DY78 有一个短基序 FPCAP,它可能通过与 EVH1(果蝇启用 (Ena)/血管扩张剂刺激的磷蛋白 (VASP) 同源性 1)域的相互作用介导与宿主细胞骨架的相互作用。尽管 Q4DY78 缺乏钙结合基序,但它的折叠类似于真核钙结合蛋白,如钙调蛋白、钙调蛋白和 polcacin Bet V4。我们将这种新型蛋白质表征为具有钙结合折叠而没有结合钙的能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号