Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-03-05 , DOI: 10.1016/j.cej.2021.129236 Luis E. Arteaga-Pérez , Raydel Manrique , Francisca Castillo-Puchi , Maray Ortega , Camila Bertiola , Andy Pérez , Romel Jiménez
|
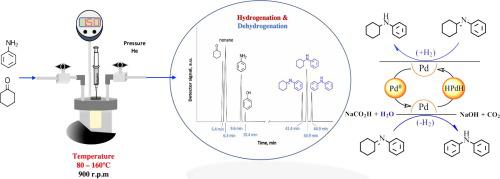
The liquid phase amination of the cyclohexanone (CyO) with aniline (PhNH2) was studied on a Pd/C catalyst using NaCO2H as a H-donor. Catalytic tests were performed in batch reactors by varying reactant concentrations (0, 0.1, 0.2 and 0.4 mol/L), the temperature (80–160 °C) and H2-equivalents availability (0, 2 and 4 H2-equivalents with respect to CyO). The results indicate that, the use of NaCO2H barely affect the reactivity (measured as initial reaction rate) while it does influence the selectivity to secondary amines. This effect was ascribed to the limited effectivity of the H2 transfer, which was controlled by the water formed after the condensation of the CyO with the PhNH2, hydrogen solubility in toluene, and the mass transfer from the liquid to the catalyst surface. The experimental observations were consistent with a multi-step mechanism consisting in: a first step of condensation of the ketone and the amine to form an imine, followed by the disproportionation of the imine into secondary amines. The kinetic measurements at initial rate conditions were well interpreted by a Langmuir Hinshelwood (L-H) kinetic model, which suggest that the surface reaction to form the intermediary imine is the rate limiting step (R.L.S).
中文翻译:

碳载钯上一锅环己酮至仲胺的胺化:揭示反应机理和动力学
使用NaCO 2 H作为氢供体,在Pd / C催化剂上研究了环己酮(CyO)与苯胺(PhNH 2)的液相胺化。在分批反应器中通过改变反应物浓度(0、0.1、0.2和0.4 mol / L),温度(80-160°C)和H 2-当量可用性(0、2和4 H 2-当量,其中关于CyO)。结果表明,使用NaCO 2 H几乎不会影响反应性(以初始反应速率衡量),但确实会影响对仲胺的选择性。这种作用归因于H 2转移的有限效率,这是由CyO与PhNH缩合后形成的水控制的。如图2所示,氢在甲苯中的溶解度以及从液体到催化剂表面的传质。实验观察结果与包括以下步骤的多步机理一致:第一步是将酮和胺缩合形成亚胺,然后将亚胺歧化成仲胺。Langmuir Hinshelwood(LH)动力学模型很好地解释了初始速率条件下的动力学测量结果,该模型表明形成中间亚胺的表面反应是速率限制步骤(RLS)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号