当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fourier-Transform Infrared Analysis of the Dehydration Mechanism of Mg(OH)2 and Chemically Modified Mg(OH)2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-03-04 , DOI: 10.1021/acs.jpcc.0c08696 Ryo Kurosawa 1 , Masato Takeuchi 2 , Junichi Ryu 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-03-04 , DOI: 10.1021/acs.jpcc.0c08696 Ryo Kurosawa 1 , Masato Takeuchi 2 , Junichi Ryu 1
Affiliation
|
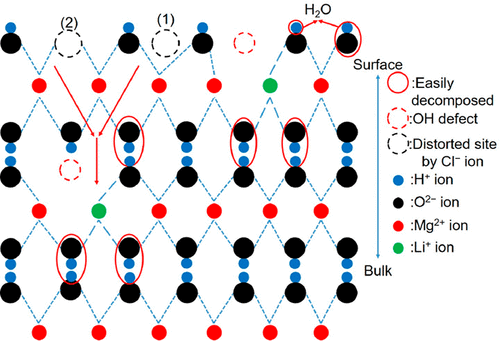
Mg(OH)2 is a medium-temperature chemical heat storage material. The addition of LiCl and/or LiOH to Mg(OH)2 has been reported to promote the dehydration of Mg(OH)2. However, the mechanism underlying the effect of the addition of Li compounds on the dehydration of Mg(OH)2 has not yet been elucidated. This study focused on the dehydration of pure Mg(OH)2 and Mg(OH)2 with added LiCl and/or LiOH. We analyzed the role of the Li compounds in the dehydration of Mg(OH)2 using X-ray diffraction and Fourier-transform infrared (FT-IR) spectroscopy. Our FT-IR measurements assigned the peaks of the bulk OH groups of Mg(OH)2, which are not referred to in previous studies. FT-IR measurements revealed that the surface state of Mg(OH)2 with added LiOH with a Mg(OH)2:LiOH mole rate of 100:20 was similar to that of MgO. This indicated that the addition of LiOH to Mg(OH)2 affected the dehydration of the Mg(OH)2 surface. Moreover, we confirmed that LiCl affected the dehydration of the bulk Mg(OH)2 phase. Using these results, we proposed possible mechanisms for the effects of the addition of LiCl and/or LiOH on the dehydration of Mg(OH)2. Lastly, we discuss the reason for the Li compounds promoting the dehydration of Mg(OH)2.
中文翻译:

Mg(OH)2和化学改性的Mg(OH)2脱水机理的傅里叶变换红外分析
Mg(OH)2是中温化学蓄热材料。据报道,向Mg(OH)2中添加LiCl和/或LiOH可促进Mg(OH)2的脱水。但是,尚未阐明添加Li化合物对Mg(OH)2脱水的影响的机理。这项研究的重点是通过添加LiCl和/或LiOH对纯Mg(OH)2和Mg(OH)2进行脱水。我们使用X射线衍射和傅里叶变换红外(FT-IR)光谱分析了Li化合物在Mg(OH)2脱水中的作用。我们的FT-IR测量指定了Mg(OH)2的整体OH基团的峰,以前的研究中未提及。FT-IR测量显示的Mg(OH)的表面状态2与加入LiOH和Mg(OH)2:100的LiOH摩尔率:20相似,MgO构成。这表明向Mg(OH)2中添加LiOH影响了Mg(OH)2表面的脱水。此外,我们证实LiCl影响了块状Mg(OH)2相的脱水。利用这些结果,我们提出了添加LiCl和/或LiOH对Mg(OH)2脱水的影响的可能机理。最后,我们讨论了Li化合物促进Mg(OH)2脱水的原因。
更新日期:2021-03-18
中文翻译:

Mg(OH)2和化学改性的Mg(OH)2脱水机理的傅里叶变换红外分析
Mg(OH)2是中温化学蓄热材料。据报道,向Mg(OH)2中添加LiCl和/或LiOH可促进Mg(OH)2的脱水。但是,尚未阐明添加Li化合物对Mg(OH)2脱水的影响的机理。这项研究的重点是通过添加LiCl和/或LiOH对纯Mg(OH)2和Mg(OH)2进行脱水。我们使用X射线衍射和傅里叶变换红外(FT-IR)光谱分析了Li化合物在Mg(OH)2脱水中的作用。我们的FT-IR测量指定了Mg(OH)2的整体OH基团的峰,以前的研究中未提及。FT-IR测量显示的Mg(OH)的表面状态2与加入LiOH和Mg(OH)2:100的LiOH摩尔率:20相似,MgO构成。这表明向Mg(OH)2中添加LiOH影响了Mg(OH)2表面的脱水。此外,我们证实LiCl影响了块状Mg(OH)2相的脱水。利用这些结果,我们提出了添加LiCl和/或LiOH对Mg(OH)2脱水的影响的可能机理。最后,我们讨论了Li化合物促进Mg(OH)2脱水的原因。










































 京公网安备 11010802027423号
京公网安备 11010802027423号