当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chitin Deacetylation Using Deep Eutectic Solvents: Ab Initio-Supported Process Optimization
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-03-05 , DOI: 10.1021/acssuschemeng.0c08976 Filipa A. Vicente 1 , Matej Huš 1, 2 , Blaž Likozar 1 , Uroš Novak 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-03-05 , DOI: 10.1021/acssuschemeng.0c08976 Filipa A. Vicente 1 , Matej Huš 1, 2 , Blaž Likozar 1 , Uroš Novak 1
Affiliation
|
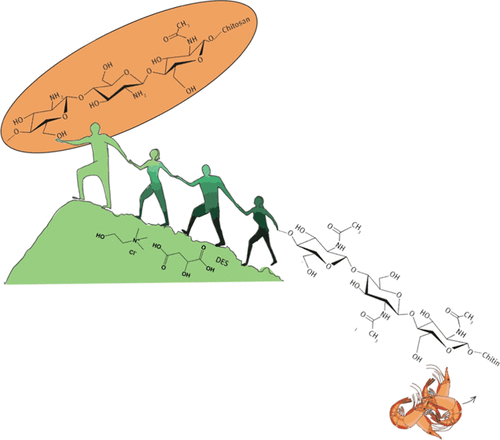
Chitin is the most abundant marine biopolymer, being recovered during the shell biorefining of crustacean shell waste. In its native form, chitin displays a poor reactivity and solubility in most solvents due to its extensive hydrogen bonding. This can be overcome by deacetylation. However, this process requires a high concentration of acids or bases at high temperatures, forming large amounts of toxic waste. Herein, we report on the first deacetylation with deep eutectic solvents (DESs) as an environmentally friendly alternative, requiring only mild reaction conditions. Biocompatible DESs are efficient in disturbing the native hydrogen-bonding network of chitin, readily dissolving it. First, quantum chemical calculations have been performed to evaluate the feasibility of different DESs to perform chitin deacetylation by studying their mechanism. Comparing these with the calculated barriers for garden-variety alkaline/acidic hydrolysis, which are known to proceed, prospective DESs were identified with barriers around 25 kcal·mol–1 or lower. Based on density functional theory results, an experimental screening of 10 distinct DESs for chitin deacetylation followed. The most promising DESs were identified as K2CO3:glycerol (K2CO3:G), choline chloride:acetic acid ([Ch]Cl:AA), and choline chloride:malic acid ([Ch]Cl:MA) and were subjected to further optimization with respect to the water content, process duration, and temperature. Ultimately, [Ch]Cl:MA showed the best results, yielding a degree of deacetylation (DDA) of 40% after 24 h of reaction at 120 °C, which falls slightly behind the threshold value (50%) for chitin to be considered chitosan. Further quantum chemical calculations were performed to elucidate the mechanism. Upon the removal of 40% N-acetyl groups from the chitin structure, its reactivity was considerably improved.
中文翻译:

使用深共晶溶剂的甲壳质脱乙酰化:从头开始支持的工艺优化
甲壳质是最丰富的海洋生物聚合物,在甲壳类贝壳废料的壳生物精制过程中被回收。几丁质以其天然形式由于其广泛的氢键作用而在大多数溶剂中显示出较差的反应性和溶解性。这可以通过脱乙酰作用来克服。但是,该方法需要高温下高浓度的酸或碱,形成大量的有毒废物。在此,我们报道了使用深共熔溶剂(DESs)进行的第一个脱乙酰反应,这是一种环境友好的替代方法,仅要求温和的反应条件。生物相容性DES可有效干扰几丁质的天然氢键网络,并易于溶解。首先,已经进行了量子化学计算,通过研究它们的机理来评估不同DES进行几丁质脱乙酰化的可行性。–1或更低。基于密度泛函理论的结果,随后进行了10种不同DES对几丁质脱乙酰化的实验筛选。最有前途的DES被确定为K 2 CO 3:甘油(K 2 CO 3:G),氯化胆碱:乙酸([Ch] Cl:AA)和氯化胆碱:苹果酸([Ch] Cl:MA),并对其水含量,处理时间和温度进行了进一步优化。最终,[Ch] Cl:MA表现出最好的结果,在120°C下反应24小时后,脱乙酰度(DDA)达到40%,略低于要考虑的几丁质的阈值(50%)壳聚糖。进行了进一步的量子化学计算以阐明其机理。从几丁质结构中除去40%的N-乙酰基后,其反应性大大提高。
更新日期:2021-03-15
中文翻译:

使用深共晶溶剂的甲壳质脱乙酰化:从头开始支持的工艺优化
甲壳质是最丰富的海洋生物聚合物,在甲壳类贝壳废料的壳生物精制过程中被回收。几丁质以其天然形式由于其广泛的氢键作用而在大多数溶剂中显示出较差的反应性和溶解性。这可以通过脱乙酰作用来克服。但是,该方法需要高温下高浓度的酸或碱,形成大量的有毒废物。在此,我们报道了使用深共熔溶剂(DESs)进行的第一个脱乙酰反应,这是一种环境友好的替代方法,仅要求温和的反应条件。生物相容性DES可有效干扰几丁质的天然氢键网络,并易于溶解。首先,已经进行了量子化学计算,通过研究它们的机理来评估不同DES进行几丁质脱乙酰化的可行性。–1或更低。基于密度泛函理论的结果,随后进行了10种不同DES对几丁质脱乙酰化的实验筛选。最有前途的DES被确定为K 2 CO 3:甘油(K 2 CO 3:G),氯化胆碱:乙酸([Ch] Cl:AA)和氯化胆碱:苹果酸([Ch] Cl:MA),并对其水含量,处理时间和温度进行了进一步优化。最终,[Ch] Cl:MA表现出最好的结果,在120°C下反应24小时后,脱乙酰度(DDA)达到40%,略低于要考虑的几丁质的阈值(50%)壳聚糖。进行了进一步的量子化学计算以阐明其机理。从几丁质结构中除去40%的N-乙酰基后,其反应性大大提高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号