Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neutrophil Elastase and Proteinase 3 Cleavage Sites Are Adjacent to the Polybasic Sequence within the Proteolytic Sensitive Activation Loop of the SARS-CoV-2 Spike Protein
ACS Omega ( IF 3.7 ) Pub Date : 2021-03-05 , DOI: 10.1021/acsomega.1c00363 Zhadyra Mustafa 1 , Anuar Zhanapiya 1 , Hubert Kalbacher 2 , Timo Burster 1
ACS Omega ( IF 3.7 ) Pub Date : 2021-03-05 , DOI: 10.1021/acsomega.1c00363 Zhadyra Mustafa 1 , Anuar Zhanapiya 1 , Hubert Kalbacher 2 , Timo Burster 1
Affiliation
|
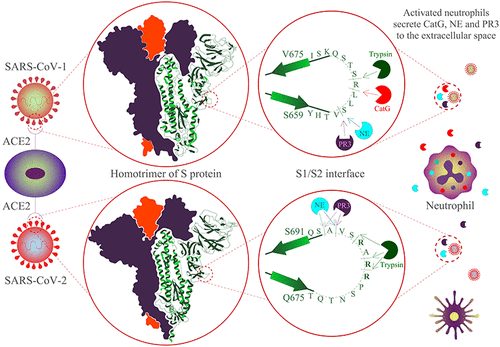
Serine proteases neutrophil elastase (NE), protease 3 (PR3), cathepsin G (CatG), and neutrophil serine protease 4 (NSP4) are released by activated neutrophils swarming around the place of pathogen invasion to provoke an immune response. However, uncontrolled proteolytic activity of proteases results in various human diseases, including cardiovascular diseases, thrombosis, and autoimmunity. In addition, proteases can be hijacked by several viruses to prime virus-derived surface proteins and evade immune detection by entering into the host cell. Indeed, porcine elastase increases the suitability of host cells to be infected by SARS-CoV-1. We compared the cleavage sites of human NE, PR3, and CatG as well as porcine-derived trypsin within the amino acid sequence of the proteolytic sensitive activation loop at the interface of S1/S2 of the spike protein (S protein) of SARS-CoV-1 as well as SARS-CoV-2. As a result, NE and PR3, but not CatG, hydrolyze the scissile peptide bond adjacent to the polybasic amino acid sequence of the S1/S2 interface of SARS-CoV-2, which is distinctive from SARS-CoV-1. These findings suggest that neutrophil-derived NE and PR3 participate in priming of the S1/S2 interface during an immune response.
中文翻译:

中性粒细胞弹性蛋白酶和蛋白酶3切割位点与SARS-CoV-2穗状蛋白的蛋白水解敏感性激活环内的多元序列相邻。
丝氨酸蛋白酶中性粒细胞弹性蛋白酶(NE),蛋白酶3(PR3),组织蛋白酶G(CatG)和中性粒细胞丝氨酸蛋白酶4(NSP4)是通过在病原体入侵的地方聚集的活化的中性粒细胞释放的,从而激发免疫应答。然而,蛋白酶的不受控制的蛋白水解活性导致多种人类疾病,包括心血管疾病,血栓形成和自身免疫。此外,蛋白酶可以被几种病毒劫持,从而引发病毒衍生的表面蛋白,并通过进入宿主细胞逃避免疫检测。实际上,猪弹性蛋白酶增加了宿主细胞被SARS-CoV-1感染的适应性。我们比较了人类NE,PR3,在SARS-CoV-1和SARS-CoV-2的刺突蛋白(S蛋白)的S1 / S2的界面上,蛋白水解敏感性激活环的氨基酸序列中包含CatG以及猪源的胰蛋白酶。结果,NE和PR3而非CatG水解了与SARS-CoV-1不同的与SARS-CoV-2的S1 / S2界面的多元氨基酸序列相邻的易裂肽键。这些发现表明,在免疫应答过程中,中性粒细胞来源的NE和PR3参与了S1 / S2接口的启动。
更新日期:2021-03-16
中文翻译:

中性粒细胞弹性蛋白酶和蛋白酶3切割位点与SARS-CoV-2穗状蛋白的蛋白水解敏感性激活环内的多元序列相邻。
丝氨酸蛋白酶中性粒细胞弹性蛋白酶(NE),蛋白酶3(PR3),组织蛋白酶G(CatG)和中性粒细胞丝氨酸蛋白酶4(NSP4)是通过在病原体入侵的地方聚集的活化的中性粒细胞释放的,从而激发免疫应答。然而,蛋白酶的不受控制的蛋白水解活性导致多种人类疾病,包括心血管疾病,血栓形成和自身免疫。此外,蛋白酶可以被几种病毒劫持,从而引发病毒衍生的表面蛋白,并通过进入宿主细胞逃避免疫检测。实际上,猪弹性蛋白酶增加了宿主细胞被SARS-CoV-1感染的适应性。我们比较了人类NE,PR3,在SARS-CoV-1和SARS-CoV-2的刺突蛋白(S蛋白)的S1 / S2的界面上,蛋白水解敏感性激活环的氨基酸序列中包含CatG以及猪源的胰蛋白酶。结果,NE和PR3而非CatG水解了与SARS-CoV-1不同的与SARS-CoV-2的S1 / S2界面的多元氨基酸序列相邻的易裂肽键。这些发现表明,在免疫应答过程中,中性粒细胞来源的NE和PR3参与了S1 / S2接口的启动。











































 京公网安备 11010802027423号
京公网安备 11010802027423号