Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Are Ionic Liquids Suitable for Suppressing Coal Spontaneous Combustion?
ACS Omega ( IF 3.7 ) Pub Date : 2021-03-04 , DOI: 10.1021/acsomega.0c05504 Xuanxuan Huang 1 , Yongliang Xu 1, 2, 3 , Yan Wang 1, 2, 3 , Yao Li 1, 2, 3 , Lanyun Wang 1, 2, 3 , Zhengyan Wu 4
ACS Omega ( IF 3.7 ) Pub Date : 2021-03-04 , DOI: 10.1021/acsomega.0c05504 Xuanxuan Huang 1 , Yongliang Xu 1, 2, 3 , Yan Wang 1, 2, 3 , Yao Li 1, 2, 3 , Lanyun Wang 1, 2, 3 , Zhengyan Wu 4
Affiliation
|
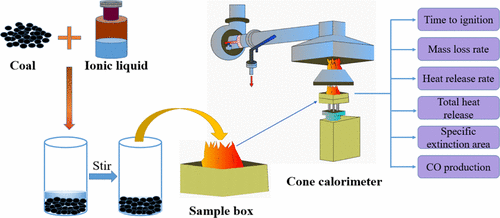
Due to the reported fact that the active functional groups in coal can be dissolved and destroyed by ionic liquids, it is expected that the spontaneous combustion of coal can be affected from a thermodynamic perspective. However, ionic liquids with different thermal stabilities have distinct influences on coal combustion. Here, the thermal stability of long-flame coal in the presence of five pure ionic liquids ([Bmim][BF4], [Bmim][Ac], [Bmim][NO3], [Hoemim][BF4], and [Pmim][BF4]) was analyzed by thermogravimetric analysis, and the flammability of the raw coal, pure ionic liquids, and coal–IL mixtures (mass ratio of 1:1) were tested using a cone calorimeter according to the indexes of the time to ignition (TTI), mass loss rate (MLR), heat release rate (HRR), total heat release rate (THR), specific extinction area (SEA), and CO production. It is shown that the TTIs of mixtures containing coal-[Bmim][BF4], coal-[Hoemim][BF4], and coal-[Pmim][BF4] are relatively long, and the MLR, HRR, THR, and SEA values are relatively low, indicating that these fluorine-containing ionic liquids have a better flame-retardant effect than the other two fluorine-free ones, which may be ascribed to their similar role to halogen inhibitors. In addition, the endothermic process of [Bmim][BF4], [Hoemim][BF4], and [Pmim][BF4] can reduce the temperature of the coal surface and delay the ignition time of coal. In contrast, the TTI of coal-[Bmim][NO3] and coal-[Bmim][Ac] mixtures is much shorter than that of coal alone, and the MLR, HRR, and THR values are larger. This may be caused by the poor thermal stability of the two nonfluorine ionic liquids that began to decompose and release heat prior to coal, providing a large amount of heat for the low-temperature oxidation of coal and thus accelerating coal oxidation and combustion. Although the F-containing ionic liquids show the ability to inhibit spontaneous combustion of coal to some extent, their organic cations are potentially combustible and release large amounts of heat, smoke, and CO under high temperatures.
中文翻译:

离子液体是否适合抑制煤自燃?
由于已报道的事实,煤中的活性官能团可被离子液体溶解和破坏,因此从热力学的角度来看,预计煤的自燃会受到影响。但是,具有不同热稳定性的离子液体对煤燃烧有明显的影响。在此,在五种纯离子液体([Bmim] [BF 4 ],[Bmim] [Ac],[Bmim] [NO 3 ],[Hoemim] [BF 4 ],和[Pmim] [BF 4])通过热重分析进行分析,并根据点火时间(TTI)的指标,使用锥形量热仪测试了原煤,纯离子液体和煤-IL混合物(质量比为1:1)的可燃性。 ),质量损失率(MLR),放热率(HRR),总放热率(THR),特定消光面积(SEA)和一氧化碳产生量。结果表明,煤-[Bmim] [BF 4 ],煤-[Hoemim] [BF 4 ]和煤-[Pmim] [BF 4的混合物的TTIs]相对较长,并且MLR,HRR,THR和SEA值相对较低,表明这些含氟离子液体的阻燃效果比其他两种不含氟的离子液体更好。与卤素抑制剂的作用相似。另外,[Bmim] [BF 4 ],[Hoemim] [BF 4 ]和[Pmim] [BF 4 ]的吸热过程可以降低煤表面的温度并延迟煤的着火时间。相比之下,煤[Bmim] [NO 3的TTI]和煤-[Bmim] [Ac]混合物要比单独的煤短得多,并且MLR,HRR和THR值较大。这可能是由于两种非氟离子液体的热稳定性差,它们在燃煤之前开始分解并释放热量,为燃煤的低温氧化提供了大量的热量,从而加速了煤的氧化和燃烧。尽管含F离子液体显示出一定程度的抑制煤自燃的能力,但它们的有机阳离子可能会燃烧,并在高温下释放大量的热,烟和CO。
更新日期:2021-03-16
中文翻译:

离子液体是否适合抑制煤自燃?
由于已报道的事实,煤中的活性官能团可被离子液体溶解和破坏,因此从热力学的角度来看,预计煤的自燃会受到影响。但是,具有不同热稳定性的离子液体对煤燃烧有明显的影响。在此,在五种纯离子液体([Bmim] [BF 4 ],[Bmim] [Ac],[Bmim] [NO 3 ],[Hoemim] [BF 4 ],和[Pmim] [BF 4])通过热重分析进行分析,并根据点火时间(TTI)的指标,使用锥形量热仪测试了原煤,纯离子液体和煤-IL混合物(质量比为1:1)的可燃性。 ),质量损失率(MLR),放热率(HRR),总放热率(THR),特定消光面积(SEA)和一氧化碳产生量。结果表明,煤-[Bmim] [BF 4 ],煤-[Hoemim] [BF 4 ]和煤-[Pmim] [BF 4的混合物的TTIs]相对较长,并且MLR,HRR,THR和SEA值相对较低,表明这些含氟离子液体的阻燃效果比其他两种不含氟的离子液体更好。与卤素抑制剂的作用相似。另外,[Bmim] [BF 4 ],[Hoemim] [BF 4 ]和[Pmim] [BF 4 ]的吸热过程可以降低煤表面的温度并延迟煤的着火时间。相比之下,煤[Bmim] [NO 3的TTI]和煤-[Bmim] [Ac]混合物要比单独的煤短得多,并且MLR,HRR和THR值较大。这可能是由于两种非氟离子液体的热稳定性差,它们在燃煤之前开始分解并释放热量,为燃煤的低温氧化提供了大量的热量,从而加速了煤的氧化和燃烧。尽管含F离子液体显示出一定程度的抑制煤自燃的能力,但它们的有机阳离子可能会燃烧,并在高温下释放大量的热,烟和CO。











































 京公网安备 11010802027423号
京公网安备 11010802027423号