当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Density and Volumetric Behavior of Ternary CO2 + n-Decane + cis-Decalin (or + trans-Decalin) Mixtures at High Pressure and High Temperature
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-03 , DOI: 10.1021/acs.jced.0c00989 Angélica Maria Chacón Valero 1 , Caiuã Araújo Alves 1 , Filipe Xavier Feitosa 1 , Hosiberto Batista de Sant’Ana 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-03 , DOI: 10.1021/acs.jced.0c00989 Angélica Maria Chacón Valero 1 , Caiuã Araújo Alves 1 , Filipe Xavier Feitosa 1 , Hosiberto Batista de Sant’Ana 1
Affiliation
|
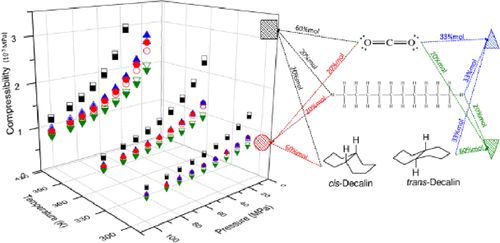
This paper presents density experimental data for pure cis-decalin and trans-decalin, along with carbon dioxide + n-decane + cis-decalin (or + trans-decalin) ternary systems for a temperature from 298.15 to 423.15 K up to 100 MPa by using a vibrating tube densitometer. Ternary mixture compositions have been chosen to cover four different regions as follows: (i) high CO2 content (xCO2 = 0.60, xn-decane = 0.20, and xdecalin = 0.20), (ii) high alkane content (xCO2 = 0.20, xn-decane = 0.60, and xdecalin = 0.20), (iii) high aromatic content (xCO2 = 0.20, xn-decane = 0.20, and xdecalin = 0.60), and an equimolar content (xCO2 = 0.33, xn-decane = 0.33, and xdecalin = 0.33). Excess molar volumes were calculated for both ternary systems, showing negative values over the entire composition range. Additionally, for both systems, experimental density data were correlated to the modified Tammann–Tait equation as a function of temperature and pressure. The adjusted equation presented a maximum deviation (MD) of 1.72%. Moreover, isothermal compressibility and isobaric thermal expansivity were calculated from the experimental density data. It was observed for both ternary systems that above 65 MPa, there is a negligible effect of pressure on isothermal compressibility regardless of the ternary system studied. For isobaric thermal expansivity, temperature dependence was found to be important at pressures below 45 MPa, while above 65 MPa is independent of temperature.
中文翻译:

高温高压下三元CO 2 +正癸烷+顺式十氢化萘(或反式十氢化萘)混合物的密度和体积行为
本文介绍了在298.15至423.15 K的温度,最高100 MPa的温度下,纯顺式十氢萘和反式十氢萘以及二氧化碳+正癸烷+顺式十氢萘(或+反式十氢萘)三元体系的密度实验数据。使用振动管密度计。选择三元混合物组成以覆盖四个不同区域,如下所示:(i)高CO 2含量(x CO2 = 0.60,x n-癸烷= 0.20 ,x decalin = 0.20),(ii)高烷烃含量(x CO2 = 0.20,x n-癸烷= 0.60,x萘烷= 0.20),(iii)高芳烃含量(x CO2 = 0.20,x n-癸烷= 0.20,x decalin = 0.60)和等摩尔含量(x CO2 = 0.33,x n-癸烷= 0.33,x十氢化萘= 0.33)。计算出两个三元体系的过量摩尔体积,在整个组成范围内均显示负值。另外,对于这两个系统,实验密度数据均与温度和压力的函数与修正的Tammann-Tait方程相关。调整后的方程式的最大偏差(MD)为1.72%。此外,从实验密度数据计算出等温压缩率和等压热膨胀率。观察到对于两种三元系统,高于65 MPa的压力对等温压缩率的影响都可以忽略不计,而与所研究的三元系统无关。对于等压热膨胀系数,发现温度低于45 MPa时温度依赖性很重要,而高于65 MPa则与温度无关。
更新日期:2021-04-08
中文翻译:

高温高压下三元CO 2 +正癸烷+顺式十氢化萘(或反式十氢化萘)混合物的密度和体积行为
本文介绍了在298.15至423.15 K的温度,最高100 MPa的温度下,纯顺式十氢萘和反式十氢萘以及二氧化碳+正癸烷+顺式十氢萘(或+反式十氢萘)三元体系的密度实验数据。使用振动管密度计。选择三元混合物组成以覆盖四个不同区域,如下所示:(i)高CO 2含量(x CO2 = 0.60,x n-癸烷= 0.20 ,x decalin = 0.20),(ii)高烷烃含量(x CO2 = 0.20,x n-癸烷= 0.60,x萘烷= 0.20),(iii)高芳烃含量(x CO2 = 0.20,x n-癸烷= 0.20,x decalin = 0.60)和等摩尔含量(x CO2 = 0.33,x n-癸烷= 0.33,x十氢化萘= 0.33)。计算出两个三元体系的过量摩尔体积,在整个组成范围内均显示负值。另外,对于这两个系统,实验密度数据均与温度和压力的函数与修正的Tammann-Tait方程相关。调整后的方程式的最大偏差(MD)为1.72%。此外,从实验密度数据计算出等温压缩率和等压热膨胀率。观察到对于两种三元系统,高于65 MPa的压力对等温压缩率的影响都可以忽略不计,而与所研究的三元系统无关。对于等压热膨胀系数,发现温度低于45 MPa时温度依赖性很重要,而高于65 MPa则与温度无关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号