Intermetallics ( IF 4.3 ) Pub Date : 2021-03-04 , DOI: 10.1016/j.intermet.2021.107160 Nadezhda S. Smirnova , Evgeny V. Khramov , Igor P. Stolarov , Ilya A. Yakushev , Galina N. Baeva , Galina O. Bragina , Ekaterina V. Belova , Arcady V. Ishchenko , Anna S. Popova , Yan V. Zubavichus , Michael N. Vargaftik , Aleksander Y. Stakheev
|
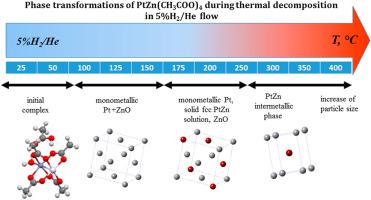
Phase transformations of a newly synthesized heterobimetallic ZnPt(OOCMe)4(H2O)(MeCOOH)2 acetate in a chemically reductive atmosphere at mildly elevated temperatures are thoroughly studied in order to optimize conditions for the formation of nanostructured PtZn intermetallic compound. According to XAFS and XRD data, the first stage of reductive thermolysis is the reduction of the noble metal, while zinc remains in an oxygen environment. At the second stage the reduction of Zn and the formation of the bimetallic solid solution with the fcc lattice occur. It is shown that recrystallization of solid solution to ordered PtZn intermetallic compound with the tetragonal structure occurs in a narrow temperature range of 250–275 °C. Based on these results, the optimum reduction temperature for the preparation of supported bimetallic Pt–Zn/Al2O3 catalyst was determined to be 300 °C. Results of catalytic tests of the supported material are reported.
中文翻译:

纳米结构的PtZn金属间化合物:由PtZn(CH 3 COO)4分子前体形成的受控结构和催化性能测试
新合成的异双金属ZnPt(OOCMe)4(H 2 O)(MeCOOH)2的相变为了优化形成纳米结构的PtZn金属间化合物的条件,对在化学还原性气氛中在适度升高的温度下的乙酸盐进行了彻底的研究。根据XAFS和XRD数据,还原性热分解的第一步是还原贵金属,而锌则保留在氧气环境中。在第二阶段,发生锌的还原和具有fcc晶格的双金属固溶体的形成。结果表明,在250-275°C的较窄温度范围内,固溶体重结晶为具有四方结构的有序PtZn金属间化合物。根据这些结果,制备负载型双金属Pt-Zn / Al 2 O 3的最佳还原温度确定催化剂为300℃。报告了载体材料的催化测试结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号