Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-03-03 , DOI: 10.1016/j.jhazmat.2021.125563 Yihao Li , Lu Jiang , Rui Wang , Pingxiao Wu , Juan Liu , Shanshan Yang , Jiahao Liang , Guining Lu , Nengwu Zhu
|
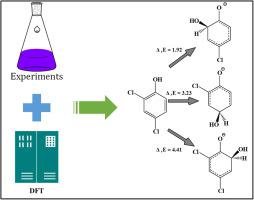
In this paper, Phenol, 4-Chlorophenol (4-CP), 2,4-Dichlorophenol (2,4-DCP) and 2,4,6-Trichlorophenol (2,4,6-TCP) were selected as model pollutants to explore the oxidant mechanism by ferrate (Fe(VI)). The reactions between ferrate (1000 μM) and four phenolic compounds (100 μM) were conformed to the second-order reaction kinetics at pH 9.2, and the order of kobs followed as: k4-CP (129 M−1 s−1) > k2,4-DCP (96 M−1 s−1) > k2,4,6-TCP (44 M−1 s−1) > kPhenol (12 M−1 s−1). Meanwhile, the degradation rates of all four compounds by Fe(VI) increased with increased pH (3.1–9.2). A total of 14 degradation products were identified by Liquid chromatography-Time-of-Flight-Mass Spectrometry (LC-TOF-MS), and two pathways including hydroxylation of benzene ring and substitution of chlorine atom were proposed. Hydroxyl radicals, played a vital role during the degradation of phenolic compounds. Moreover, density functional theory calculations were used to explore the degradation mechanisms. The results showed that the hydroxyl radical was more favorable to substitute chlorine atom than hydrogen atom, and the substitution on ortho-position was more favorable than para-position for all four compounds. The findings of this study could greatly improve our understanding on the degradation mechanism of chlorophenol-like compounds by Fe(VI) for environmental remediation.
中文翻译:

密度泛函理论辅助高铁酸盐(VI)对酚类化合物的动力学和机理
本文选择了苯酚,4-氯苯酚(4-CP),2,4-二氯苯酚(2,4-DCP)和2,4,6-三氯苯酚(2,4,6-TCP)作为模型污染物探索高铁酸盐(Fe(VI))的氧化机理。高铁酸盐(1000μM)与四种酚类化合物(100μM)之间的反应符合pH 9.2下的二级反应动力学,k obs的顺序为:k 4-CP(129 M -1 s -1)> k 2,4-DCP(96 M -1 s -1)> k 2,4,6-TCP(44 M -1 s -1)> k苯酚(12 M -1 s-1)。同时,Fe(VI)对这四种化合物的降解率均随pH值的升高(3.1-9.2)而增加。通过液相色谱-飞行时间质谱(LC-TOF-MS)鉴定出总共14种降解产物,并提出了苯环羟基化和氯原子取代的两条途径。羟基在酚类化合物的降解过程中起着至关重要的作用。此外,使用密度泛函理论计算来探索降解机理。结果表明,对于所有四种化合物,羟基自由基都比氢原子更易于取代氯原子,邻位取代对位更有利。











































 京公网安备 11010802027423号
京公网安备 11010802027423号