Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2021-03-03 , DOI: 10.1016/j.fluid.2021.113003 Gholamhossein Sodeifian , Ratna Surya Alwi , Fariba Razmimanesh , Kazuhiro Tamura
|
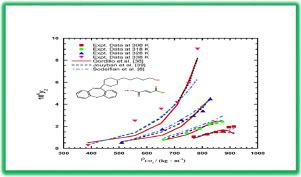
This study reports the first measurement of the solubility of Quetiapine hemifumarate (QHF, an antipsychotic drug) in supercritical carbon dioxide (ScCO2) by static method apparatus at temperatures range of 308–338 K and pressures range of 120–270 bar. The solubility of QHF varied between 0.30 10−6 and 9.03 10−6 (in mole fraction). Three types of models were selected to correlate experimental data: (1) semi-empirical models with 3 to 6 adjustable parameters developed by Chrastil, Méndenz-Santiago & Teja, Bartle, Sung-Shim, Hozhabr, Jafari, Bian, Garlapati – Madras, Keshmiri, Khansary, Gordillo, Jouyban, and Sodeifian; (2) the solution model along with Hansen parameter; and (3) the Peng Robinson's cubic equation with conventional temperature-independent mixing rule. According to the results, all types of models adequately determined the solubility data of QHF in ScCO2 with acceptable accuracy. However, among all empirical models, Garlapati – Madras model led to the best results (AARD of 7.29); at the same time, the solution model with the Hansen parameter yielded a good correlation for the solubility of 12 compounds (mean AARD of 6.54). Furthermore, thermodynamic quantities of solvation and sublimation enthalpies were estimated.
中文翻译:

的溶解度喹硫平半富马酸盐的实验,建模和Hansen溶解度参数应用:在超临界二氧化碳(抗精神病药)
这项研究报告了通过静态方法在308-338 K的温度范围和120-270 bar的压力范围内首次测量喹硫平半富马酸盐(QHF,一种抗精神病药物)在超临界二氧化碳(ScCO 2)中的溶解度。QHF的溶解度在0.30之间变化10 -6和9.0310 -6(以摩尔分数计)。选择了三种类型的模型来关联实验数据:(1)Chrastil,Mennz-Santiago&Teja,Bartle,Sung-Shim,Hozhabr,Jafari,Bian,Garlapati – Madras,克什米尔(Keshmiri),汉萨里(Khansary),戈迪略(Gordillo),茹伊班(Jouyban)和索德菲安(Sodeifian);(2)连同汉森参数的求解模型;(3)具有常规温度无关混合规则的Peng Robinson三次方程。根据结果,所有类型的模型都充分确定了QHF在ScCO 2中的溶解度数据可以接受的精度。然而,在所有的经验模型中,加拉帕蒂-马德拉斯模型导致了最好的结果(AARD为7.29)。同时,具有Hansen参数的溶液模型对12种化合物的溶解度具有良好的相关性(平均AARD为6.54)。此外,估计了溶剂化和升华焓的热力学量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号