Chemistry and Physics of Lipids ( IF 3.4 ) Pub Date : 2021-03-03 , DOI: 10.1016/j.chemphyslip.2021.105073 Chae Eun Heo 1 , Chae Ri Park 1 , Hugh I Kim 1
|
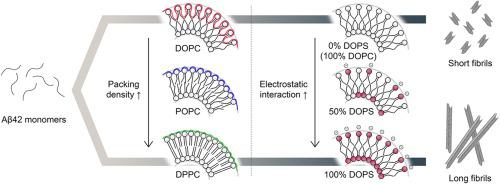
The aggregation of amyloid-β 1–42 (Aβ42) on lipid membranes is closely related to the pathology of Alzheimer’s disease (AD). Herein, we demonstrated the effect of the packing density of lipid vesicles on the Aβ42 fibrillation kinetics and fibril morphology. We used three distinct phosphatidylcholine (PC) lipids, containing different numbers of cis-double bonds in acyl chains, and therefore, a different packing density in the lipid vesicles. Our results showed that the fibrillation of Aβ42 was greatly enhanced and the formed fibrils became shorter as the number of double bonds in lipids increased. Due to the low-density characteristics of dioleoyl phosphatidylcholine (DOPC), Aβ42 monomers were able to interact with the hydrophobic acyl chain of lipids exposed to the aqueous phase, thereby inducing rapid fibrillation and short fibril morphologies. Furthermore, the effects of the anionic lipids dioleoyl phosphatidylserine (DOPS) and dioleoyl phosphatidylglycerol (DOPG), and mixed vesicles of DOPC/DOPS and DOPC/DOPG on Aβ42 fibrillations were investigated. The tight binding of Aβ42 to the lipid head groups via electrostatic interactions was able to suppress the modulation of Aβ42 fibrillations compared to accelerated fibrillations on loosely packed membranes. Our proposed mechanism regarding the influence of lipid packing density on Aβ42 fibrillations provides an advanced understanding of lipid-associated amyloid fibrillations.
中文翻译:

脂质囊泡堆积密度对Aβ42原纤维多态性的影响
淀粉样蛋白-β1-42 (Aβ42) 在脂质膜上的聚集与阿尔茨海默病 (AD) 的病理学密切相关。在此,我们证明了脂质囊泡的堆积密度对 Aβ42 纤维化动力学和纤维形态的影响。我们使用了三种不同的磷脂酰胆碱 (PC) 脂质,包含不同数量的顺式-酰基链中的双键,因此,脂质囊泡中的堆积密度不同。我们的结果表明,随着脂质中双键数量的增加,Aβ42 的原纤维化大大增强,形成的原纤维变短。由于二油酰磷脂酰胆碱 (DOPC) 的低密度特性,Aβ42 单体能够与暴露于水相的脂质的疏水酰基链相互作用,从而诱导快速原纤维形成和短原纤维形态。此外,研究了阴离子脂质二油酰磷脂酰丝氨酸 (DOPS) 和二油酰磷脂酰甘油 (DOPG) 以及 DOPC/DOPS 和 DOPC/DOPG 的混合囊泡对 Aβ42 纤维化的影响。Aβ42 与脂质头基的紧密结合通过与松散堆积膜上的加速纤维化相比,静电相互作用能够抑制 Aβ42 纤维化的调节。我们提出的关于脂质堆积密度对 Aβ42 纤维化影响的机制提供了对脂质相关淀粉样蛋白纤维化的深入理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号