Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.jacc.2020.12.058 R Scott Wright 1 , Kausik K Ray 2 , Frederick J Raal 3 , David G Kallend 4 , Mark Jaros 5 , Wolfgang Koenig 6 , Lawrence A Leiter 7 , Ulf Landmesser 8 , Gregory G Schwartz 9 , Andrew Friedman 10 , Peter L J Wijngaard 10 , Lorena Garcia Conde 11 , John J P Kastelein 12 ,
|
Background
Inclisiran is a double-stranded small interfering RNA that suppresses proprotein convertase subtilisin–kexin type 9 (PCSK9) translation in the liver, leading to sustained reductions in low-density lipoprotein cholesterol (LDL-C) and other atherogenic lipoproteins with twice-yearly dosing.
Objectives
The purpose of this study was to conduct a patient-level pooled analysis from 3 phase 3 studies of inclisiran.
Methods
Participants with heterozygous familial hypercholesterolemia (ORION-9 [Trial to Evaluate the Effect of Inclisiran Treatment on Low Density Lipoprotein Cholesterol (LDL-C) in Subjects With Heterozygous Familial Hypercholesterolemia (HeFH)]), atherosclerotic cardiovascular disease (ASCVD) (ORION-10 [Inclisiran for Participants With Atherosclerotic Cardiovascular Disease and Elevated Low-density Lipoprotein Cholesterol]), or ASCVD and ASCVD risk equivalents (ORION-11 [Inclisiran for Subjects With ASCVD or ASCVD-Risk Equivalents and Elevated Low-density Lipoprotein Cholesterol]) taking maximally tolerated statin therapy, with or without other LDL-C–lowering agents, were randomly assigned in a 1:1 ratio to receive either inclisiran or placebo, administered by subcutaneous injection on day 1, day 90, and every 6 months thereafter for 540 days. The coprimary endpoints were the placebo-corrected percentage change in LDL-C level from baseline to day 510 and the time-adjusted percentage change in LDL-C level from baseline after day 90 to day 540. Levels of other atherogenic lipoproteins and treatment-emergent adverse events were also assessed.
Results
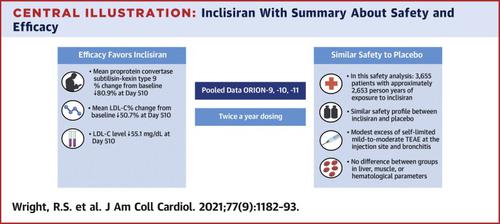
A total of 3,660 participants (n = 482, n = 1,561, and n = 1,617 from ORION-9, -10, and -11, respectively) underwent randomization. The placebo-corrected change in LDL-C with inclisiran at day 510 was −50.7% (95% confidence interval: −52.9% to −48.4%; p < 0.0001). The corresponding time-adjusted change in LDL-C was −50.5% (95% confidence interval: −52.1% to −48.9%; p < 0.0001). Safety was similar in both groups. Treatment-emergent adverse events at the injection site were more frequent with inclisiran than placebo (5.0% vs. 0.7%), but were predominantly mild, and none were severe or persistent. Liver and kidney function tests, creatine kinase values, and platelet counts did not differ between groups.
Conclusions
These pooled safety and efficacy data show that inclisiran, given twice yearly in addition to maximally tolerated statin therapy with or without other LDL-C lowering agents, is an effective, safe, and well-tolerated treatment to lower LDL-C in adults with heterozygous familial hypercholesterolemia, ASCVD, or ASCVD risk equivalents.
中文翻译:

对家族性高胆固醇血症或动脉粥样硬化患者进行 Inclisiran 试验的合并患者水平分析
背景
Inclisiran 是一种双链小干扰 RNA,可抑制肝脏中前蛋白转化酶枯草溶菌素 - kexin 9 型 (PCSK9) 的翻译,导致低密度脂蛋白胆固醇 (LDL-C) 和其他致动脉粥样硬化的脂蛋白持续降低,每年给药两次.
目标
本研究的目的是对 inclisiran 的 3 项 3 期研究进行患者水平的汇总分析。
方法
杂合子家族性高胆固醇血症 (ORION-9 [试验评估 Inclisiran 治疗对杂合子家族性高胆固醇血症 (HeFH) 患者低密度脂蛋白胆固醇 (LDL-C) 的影响])、动脉粥样硬化性心血管疾病 (ASCVD) (ORION-10) 的参与者[Inclisiran 用于动脉粥样硬化性心血管疾病和低密度脂蛋白胆固醇升高的参与者]),或 ASCVD 和 ASCVD 风险等价物(ORION-11 [Inclisiran 用于患有 ASCVD 或 ASCVD 风险等价物和升高的低密度脂蛋白胆固醇的受试者])耐受他汀类药物治疗,加或不加其他降 LDL-C 药物,以 1:1 的比例随机分配接受 inclisiran 或安慰剂,在第 1 天、第 90 天和此后每 6 个月皮下注射给药,共 540 天.共同主要终点是安慰剂校正的 LDL-C 水平从基线到第 510 天的百分比变化以及第 90 天到第 540 天后 LDL-C 水平从基线的时间调整百分比变化。还评估了不良事件。
结果
共有 3,660 名参与者(分别来自 ORION-9、-10 和 -11 的 n = 482、n = 1,561 和 n = 1,617)接受了随机化。在第 510 天使用 inclisiran 安慰剂校正的 LDL-C 变化为 -50.7%(95% 置信区间:-52.9% 至 -48.4%;p < 0.0001)。相应的时间调整后 LDL-C 变化为 -50.5%(95% 置信区间:-52.1% 至 -48.9%;p < 0.0001)。两组的安全性相似。与安慰剂相比,inclisiran 注射部位治疗中出现的不良事件更频繁(5.0% 对 0.7%),但主要是轻微的,没有严重或持续的不良事件。肝肾功能检查、肌酸激酶值和血小板计数在组间没有差异。
结论
这些汇总的安全性和有效性数据表明,除了最大耐受性他汀类药物治疗外,每年两次给予 inclisiran 联合或不联合其他 LDL-C 降低剂,是一种有效、安全且耐受良好的治疗方法,可降低患有杂合子的成人的 LDL-C。家族性高胆固醇血症、ASCVD 或 ASCVD 风险等价物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号