当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low-Temperature CO2 Thermal Reduction to Graphitic and Diamond-like Carbons Using Perovskite-Type Titanium Nanoceramics by Quasi-High-Pressure Reactions
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-03-01 , DOI: 10.1021/acssuschemeng.0c08963 Takumi Watanabe 1 , Tomonori Ohba 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2021-03-01 , DOI: 10.1021/acssuschemeng.0c08963 Takumi Watanabe 1 , Tomonori Ohba 1
Affiliation
|
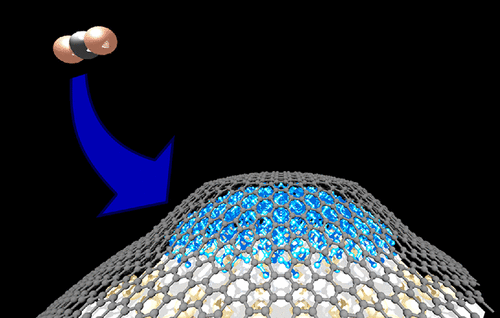
Catalytic thermal reduction of CO2 has the potential to minimize the energy requirement in carbon capture and utilization. However, this process necessitates high energy with a reactant gas, heating, and pressurization. High-performance catalysts are thus needed to transform CO2 into value-added carbon products at low energy consumption. We here demonstrate the thermal reduction of CO2 to carbon products at 700 K and ambient pressure using nanoscale perovskite-type titanium nanocatalysts. The reactivity of CO2 was exponentially increased by decreasing the particle size until 10 nm. The reaction yields calculated from the weight changes of nanocatalysts under CO2 flow were very high, i.e., between 1600 and 3300 μmol g–1 h–1 for nanocatalysts smaller than 20 nm, which were similar to those of CO2 reductions with a reactant gas into lower hydrocarbons and alcohols. A CO2 reductant was evaluated by transmission electron microscopy, chemical mapping using energy-dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy, and Raman scattering spectroscopy. Graphitic carbons were coated on 9–12 nm nanocatalysts after CO2 reduction. Graphitic carbons were mainly observed on the 12 nm nanocatalyst. A nanodiamond-like structure was partly observed on the 9 nm nanocatalyst, as well as a graphitic structure. Nanodiamond was hardly produced in the mild condition at 700 K and ambient pressure, which was normally produced above 2 GPa and 1600 K. A nanodiamond was produced by quasi-high-pressure effect in graphene and amorphous carbons, which were previously produced on nanocatalysts. The low-temperature CO2 thermal reduction using perovskite-type titanium nanocatalysts at 700 K is thus a promising approach to CO2 reduction and novel way to produce nanodiamond-like structure.
中文翻译:

低温CO 2热还原到石墨和钻石般的炭使用钙钛矿型氧化钛纳米陶瓷的准高压反应
CO 2的催化热还原具有使碳捕获和利用中的能量需求最小化的潜力。但是,该过程需要通过反应气体,加热和加压来获得高能量。因此,需要高性能的催化剂以低能耗将CO 2转化为增值的碳产品。我们在这里展示了使用纳米钙钛矿型钛纳米催化剂在700 K和环境压力下将CO 2热还原为碳产物的过程。通过减小粒径直至10 nm ,CO 2的反应性呈指数增加。由CO 2下纳米催化剂的重量变化计算反应产率流量非常高,例如,小于20 nm的纳米催化剂在1600和3300μmolg –1 h –1之间,这类似于用反应气体将CO 2还原为低级烃和醇的过程。通过透射电子显微镜,使用能量色散X射线能谱,X射线光电子能谱和拉曼散射能谱的化学作图来评估CO 2还原剂。在CO 2排放后,将石墨碳涂覆在9–12 nm纳米催化剂上减少。石墨碳主要在12 nm纳米催化剂上观察到。在9nm纳米催化剂上部分观察到纳米金刚石状结构以及石墨结构。纳米金刚石几乎没有在温和条件下在700 K和环境压力下生产,通常在2 GPa和1600 K以上的环境下生产。纳米金刚石是通过石墨烯和无定形碳的准高压作用生产的,而石墨烯和无定形碳是以前在纳米催化剂上生产的。因此,使用钙钛矿型钛纳米催化剂在700 K下进行低温CO 2热还原是一种有希望的CO 2还原方法,也是一种生产纳米金刚石状结构的新方法。
更新日期:2021-03-15
中文翻译:

低温CO 2热还原到石墨和钻石般的炭使用钙钛矿型氧化钛纳米陶瓷的准高压反应
CO 2的催化热还原具有使碳捕获和利用中的能量需求最小化的潜力。但是,该过程需要通过反应气体,加热和加压来获得高能量。因此,需要高性能的催化剂以低能耗将CO 2转化为增值的碳产品。我们在这里展示了使用纳米钙钛矿型钛纳米催化剂在700 K和环境压力下将CO 2热还原为碳产物的过程。通过减小粒径直至10 nm ,CO 2的反应性呈指数增加。由CO 2下纳米催化剂的重量变化计算反应产率流量非常高,例如,小于20 nm的纳米催化剂在1600和3300μmolg –1 h –1之间,这类似于用反应气体将CO 2还原为低级烃和醇的过程。通过透射电子显微镜,使用能量色散X射线能谱,X射线光电子能谱和拉曼散射能谱的化学作图来评估CO 2还原剂。在CO 2排放后,将石墨碳涂覆在9–12 nm纳米催化剂上减少。石墨碳主要在12 nm纳米催化剂上观察到。在9nm纳米催化剂上部分观察到纳米金刚石状结构以及石墨结构。纳米金刚石几乎没有在温和条件下在700 K和环境压力下生产,通常在2 GPa和1600 K以上的环境下生产。纳米金刚石是通过石墨烯和无定形碳的准高压作用生产的,而石墨烯和无定形碳是以前在纳米催化剂上生产的。因此,使用钙钛矿型钛纳米催化剂在700 K下进行低温CO 2热还原是一种有希望的CO 2还原方法,也是一种生产纳米金刚石状结构的新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号