当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New Phenylpropanoid Glycosides from Illicium majus and Their Radical Scavenging Activities
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2021-02-28 , DOI: 10.1002/cbdv.202001012 Fang Li 1, 2, 3 , Ting-Ting Yan 1, 2 , Ying-Ying Fu 1, 2 , Nen-Ling Zhang 4 , Lei Wang 3 , Yan-Bing Zhang 1 , Juan Du 1 , Ji-Feng Liu 1
Chemistry & Biodiversity ( IF 2.9 ) Pub Date : 2021-02-28 , DOI: 10.1002/cbdv.202001012 Fang Li 1, 2, 3 , Ting-Ting Yan 1, 2 , Ying-Ying Fu 1, 2 , Nen-Ling Zhang 4 , Lei Wang 3 , Yan-Bing Zhang 1 , Juan Du 1 , Ji-Feng Liu 1
Affiliation
|
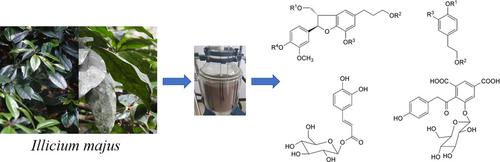
Chemical investigation of the ethanol extract of the branch and leaves of Illicium majus resulted in the isolation of four new phenylpropanoid glycosides (1–4) and one new phenolic glycoside (9), along with 13 known ones. Spectroscopic techniques were used to elucidate the structures of the new isolates such as 3‐[(2R,3S)‐7‐hydroxy‐2‐(4‐hydroxy‐3‐methoxyphenyl)‐3‐(hydroxymethyl)‐2,3‐dihydro‐1‐benzofuran‐5‐yl]propyl β‐D‐glucopyranoside (1), [(2R,3S)‐7‐hydroxy‐2‐(4‐hydroxy‐3‐methoxyphenyl)‐5‐(3‐hydroxypropyl)‐2,3‐dihydro‐1‐benzofuran‐3‐yl]methyl 2‐O‐α‐L‐rhamnopyranosyl‐β‐D‐glucopyranoside (2), [(2R,3S)‐7‐hydroxy‐2‐(4‐hydroxy‐3‐methoxyphenyl)‐5‐(3‐hydroxypropyl)‐2,3‐dihydro‐1‐benzofuran‐3‐yl]methyl 2‐O‐α‐L‐rhamnopyranosyl‐β‐D‐xylopyranoside (3), 3‐[(2R,3S)‐3‐({[2‐O‐(4‐O‐acetyl‐α‐L‐rhamnopyranosyl)‐β‐D‐xylopyranosyl]oxy}methyl)‐7‐hydroxy‐2‐(4‐hydroxy‐3‐methoxyphenyl)‐2,3‐dihydro‐1‐benzofuran‐5‐yl]propyl acetate (4), and 4‐(2‐hydroxyethyl)phenyl 3‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranoside (9). Free radical scavenging activities of the isolates were elucidated through the DPPH assay method. The most active compounds, 1‐O‐caffeoyl‐β‐D‐glucopyranose (17) and soulieana acid 1 (18), exhibited moderate radical scavenging activities (IC50=37.7±4.4 μM and IC50=97.2±3.4 μM, respectively). The antibacterial activities of the isolates against Staphylococcus aureus and Escherichia coli were also assessed, and no activity was shown at the measured concentration (<32 μg/mL).
中文翻译:

八角茴香中新的苯丙烷类糖苷及其自由基清除活性
分支和叶的乙醇提取物的化学调查八角草导致的四个新的苯丙甙(隔离1 - 4)和一个新的酚类糖苷(9)中,用13周公知的沿。使用光谱技术阐明了新分离株的结构,例如3-[(2 R,3 S)-7-羟基-2-(4-羟基-3-甲氧基苯基)-3-(羟甲基)-2,3 -二氢-1-苯并呋喃-5-基]丙基β-D-吡喃葡萄糖苷(1),[(2 R,3 S)-7-羟基-2-(4-羟基-3-甲氧基苯基)-5-(3 -羟丙基)-2,3-二氢-1-苯并呋喃-3-基]甲基2- O-α-L-鼠李糖醛糖基-β-D-吡喃葡萄糖苷(2),[(2 R,3 S)-7-羟基-2-(4-羟基-3-甲氧基苯基)-5-(3-羟基丙基)-2 ,3-二氢-1-苯并呋喃-3-基]甲基2-邻-α-L-鼠李糖基吡喃糖基-β-D-吡喃吡喃糖苷(3),3-[((2 R,3 S)-3-({[2 - ö - (4- ö乙酰基α-L-吡喃鼠李糖基)-β-d-D-吡喃木糖基]氧基}甲基)-7-羟基-2-(4-羟基-3-甲氧基苯基)-2,3-二氢乙酸1-苯并呋喃-5-基]丙酯(4)和4-(2-羟乙基)苯基3 - O -β-D-吡喃葡萄糖基-β-D-吡喃葡萄糖苷(9)。通过DPPH测定法阐明了分离物的自由基清除活性。活性最高的化合物1 - O-咖啡酰-β -D-吡喃葡萄糖(17)和香豆酸1(18)表现出中等的自由基清除活性(分别为IC 50 = 37.7±4.4μM和IC 50 = 97.2±3.4μM )。还评估了分离物对金黄色葡萄球菌和大肠杆菌的抗菌活性,在测得的浓度(<32μg/ mL)下未显示出活性。
更新日期:2021-04-14
中文翻译:

八角茴香中新的苯丙烷类糖苷及其自由基清除活性
分支和叶的乙醇提取物的化学调查八角草导致的四个新的苯丙甙(隔离1 - 4)和一个新的酚类糖苷(9)中,用13周公知的沿。使用光谱技术阐明了新分离株的结构,例如3-[(2 R,3 S)-7-羟基-2-(4-羟基-3-甲氧基苯基)-3-(羟甲基)-2,3 -二氢-1-苯并呋喃-5-基]丙基β-D-吡喃葡萄糖苷(1),[(2 R,3 S)-7-羟基-2-(4-羟基-3-甲氧基苯基)-5-(3 -羟丙基)-2,3-二氢-1-苯并呋喃-3-基]甲基2- O-α-L-鼠李糖醛糖基-β-D-吡喃葡萄糖苷(2),[(2 R,3 S)-7-羟基-2-(4-羟基-3-甲氧基苯基)-5-(3-羟基丙基)-2 ,3-二氢-1-苯并呋喃-3-基]甲基2-邻-α-L-鼠李糖基吡喃糖基-β-D-吡喃吡喃糖苷(3),3-[((2 R,3 S)-3-({[2 - ö - (4- ö乙酰基α-L-吡喃鼠李糖基)-β-d-D-吡喃木糖基]氧基}甲基)-7-羟基-2-(4-羟基-3-甲氧基苯基)-2,3-二氢乙酸1-苯并呋喃-5-基]丙酯(4)和4-(2-羟乙基)苯基3 - O -β-D-吡喃葡萄糖基-β-D-吡喃葡萄糖苷(9)。通过DPPH测定法阐明了分离物的自由基清除活性。活性最高的化合物1 - O-咖啡酰-β -D-吡喃葡萄糖(17)和香豆酸1(18)表现出中等的自由基清除活性(分别为IC 50 = 37.7±4.4μM和IC 50 = 97.2±3.4μM )。还评估了分离物对金黄色葡萄球菌和大肠杆菌的抗菌活性,在测得的浓度(<32μg/ mL)下未显示出活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号