Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual Size/Charge‐Switchable Nanocatalytic Medicine for Deep Tumor Therapy
Advanced Science ( IF 14.3 ) Pub Date : 2021-03-01 , DOI: 10.1002/advs.202002816 Wencheng Wu 1, 2 , Yinying Pu 3 , Jianlin Shi 1, 2
Advanced Science ( IF 14.3 ) Pub Date : 2021-03-01 , DOI: 10.1002/advs.202002816 Wencheng Wu 1, 2 , Yinying Pu 3 , Jianlin Shi 1, 2
Affiliation
|
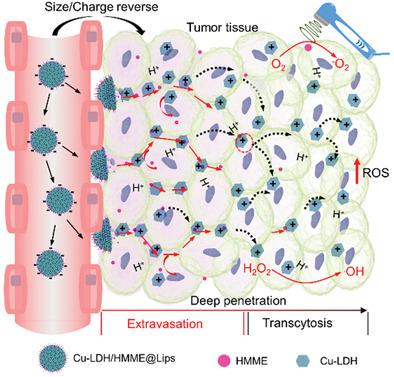
Elevating intratumoral levels of highly toxic reactive oxygen species (ROS) by nanocatalytic medicine for tumor‐specific therapy without using conventional toxic chemodrugs is recently of considerable interest, which, however, still suffers from less satisfactory therapeutic efficacy due to the relatively poor accumulation at the tumor site and largely blocked intratumoral infiltration of nanomedicines. Herein, an ultrasound (US)‐triggered dual size/charge‐switchable nanocatalytic medicine, designated as Cu‐LDH/HMME@Lips, is constructed for deep solid tumor therapy via catalytic ROS generations. The negatively charged liposome outer‐layer of the nanomedicine enables much‐prolonged blood circulation for significantly enhanced tumoral accumulation, while the positively charged Fenton‐like catalyst Cu‐LDH released from the liposome under the US stimulation demonstrates much enhanced intratumoral penetration via transcytosis. In the meantime, the co‐released sonosensitizer hematoporphyrin monomethyl ether (HMME) catalyze the singlet oxygen (1O2) generation upon the US irradiation, and deep‐tumoral infiltrated Cu‐LDH catalyzes the H2O2 decomposition to produce highly toxic hydroxyl radical (·OH) specifically within the mildly acidic tumor microenvironment (TME). The efficient intratumoral accumulation and penetration via the dual size/charge switching mechanism, and the ROS generations by both sonosensitization and Fenton‐like reactions, ensures the high therapeutic efficacy for the deep tumor therapy by the nanocatalytic medicine.
中文翻译:

用于深度肿瘤治疗的双尺寸/电荷可切换纳米催化药物
通过纳米催化药物提高肿瘤内高毒性活性氧(ROS)水平,在不使用传统有毒化学药物的情况下进行肿瘤特异性治疗,最近引起了人们的极大兴趣,然而,由于在肿瘤中的积累相对较差,其治疗效果仍然不太令人满意。肿瘤部位并在很大程度上阻断了纳米药物的瘤内浸润。在此,构建了一种超声(US)触发的双尺寸/电荷可切换纳米催化药物,称为 Cu-LDH/HMME@Lips,通过催化 ROS 生成用于深层实体瘤治疗。纳米药物的带负电的脂质体外层能够延长血液循环,从而显着增强肿瘤的积累,而带正电的类芬顿催化剂 Cu-LDH 在超声刺激下从脂质体中释放出来,通过胞吞作用大大增强了肿瘤内的渗透。同时,共同释放的声敏剂血卟啉单甲醚(HMME)在US照射下催化单线态氧(1 O 2)的产生,肿瘤深部浸润的Cu-LDH催化H 2 O 2分解产生剧毒的羟基自由基(·OH)特别存在于弱酸性肿瘤微环境(TME)中。通过双尺寸/电荷转换机制实现的高效瘤内积累和渗透,以及通过声敏和类芬顿反应产生ROS,确保了纳米催化药物对深部肿瘤治疗的高疗效。
更新日期:2021-05-05
中文翻译:

用于深度肿瘤治疗的双尺寸/电荷可切换纳米催化药物
通过纳米催化药物提高肿瘤内高毒性活性氧(ROS)水平,在不使用传统有毒化学药物的情况下进行肿瘤特异性治疗,最近引起了人们的极大兴趣,然而,由于在肿瘤中的积累相对较差,其治疗效果仍然不太令人满意。肿瘤部位并在很大程度上阻断了纳米药物的瘤内浸润。在此,构建了一种超声(US)触发的双尺寸/电荷可切换纳米催化药物,称为 Cu-LDH/HMME@Lips,通过催化 ROS 生成用于深层实体瘤治疗。纳米药物的带负电的脂质体外层能够延长血液循环,从而显着增强肿瘤的积累,而带正电的类芬顿催化剂 Cu-LDH 在超声刺激下从脂质体中释放出来,通过胞吞作用大大增强了肿瘤内的渗透。同时,共同释放的声敏剂血卟啉单甲醚(HMME)在US照射下催化单线态氧(1 O 2)的产生,肿瘤深部浸润的Cu-LDH催化H 2 O 2分解产生剧毒的羟基自由基(·OH)特别存在于弱酸性肿瘤微环境(TME)中。通过双尺寸/电荷转换机制实现的高效瘤内积累和渗透,以及通过声敏和类芬顿反应产生ROS,确保了纳米催化药物对深部肿瘤治疗的高疗效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号