Journal of Physics and Chemistry of Solids ( IF 4.3 ) Pub Date : 2021-02-27 , DOI: 10.1016/j.jpcs.2021.110020 Moussa Boudiaf , Youcef Messai , Embarek Bentouhami , Marck Schmutz , Christian Blanck , Laurent Ruhlmann , Hamza Bezzi , Latifa Tairi , Djamel Eddine Mekki
|
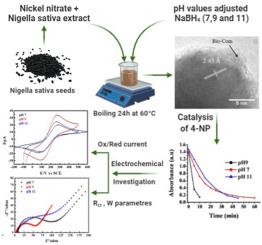
Green-synthesized NiO nanoparticles (NiONPS) have been developed using Nigella Sativa seeds extract as stabilizing agent. The pH of the solution has been modulated, by the addition of NaBH4. NiONPs have been characterized by XRD, FTIR, SEM and TEM. FTIR measurements and TEM images highlighted that NiONPs were surrounded by some biological compounds coming from the extract. The total reduction of 4-nitrophenol to 4-aminophenol corresponding to the catalytic activity of NiONPs was occurred at pH7, pH9 and pH11 in 60min, 10min and 45min respectively. These results revealed that the best catalytic activity is obtained at pH9 which is confirmed by electrocatalytical activity measurements where the resistance charge transfer corresponding is 53.7 Ω cm2 and the Warburg constant 0.0395Ωs-1/2 related a good ion diffusion, indicated consequently an increase number of electroactive sites. Thus, the carrier charges generated in the NiO mass can easily reach the surface which contribute to the reduction of 4-nitrophenol.
中文翻译:

用黑黑草提取物绿色合成NiO纳米粒子及其增强的对4-硝基苯酚降解的电催化活性
已使用苜蓿(Nigella Sativa)种子提取物作为稳定剂开发了绿色合成的NiO纳米颗粒(NiONP S)。通过添加NaBH 4可以调节溶液的pH值。NiONPs已通过XRD,FTIR,SEM和TEM进行了表征。FTIR测量和TEM图像突出显示NiONP被提取物中的一些生物化合物包围。分别在60min,10min和45min分别在pH7,pH9和pH11下发生了与NiONPs催化活性相对应的4-硝基苯酚向4-氨基苯酚的总还原。这些结果表明,在pH9时获得了最佳的催化活性,这通过电催化活性测量得到了证实,其中相应的电阻电荷转移为53.7Ωcm 2Warburg常数0.0395Ωs-1 /2与良好的离子扩散相关,因此表明电活性位点数量增加。因此,在NiO物质中产生的载流子电荷可以容易地到达表面,这有助于4-硝基苯酚的还原。











































 京公网安备 11010802027423号
京公网安备 11010802027423号