Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-02-28 , DOI: 10.1016/j.jmgm.2021.107875 Eliana K Asciutto 1 , Sergio Pantano 2 , Ignacio J General 1
|
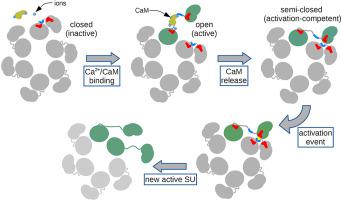
CaMKII is a protein kinase whose function is regulated by the binding of the Calcium/Calmodulin complex (Ca2+/CaM). It is a major player in the Long Term Potentiation process where it acts as a molecular switch, oscillating between inhibited and active conformations. The mechanism for the switching is thought to be initiated by Ca2+/CaM binding, which allows the trans-phosphorylation of a subunit of CaMKII by a neighboring kinase, leading to the active state of the system. A combination of all-atom and coarse-grained MD simulations with free energy calculations, led us to reveal an interplay of electrostatic forces exerted by Ca2+/CaM on CaMKII, which initiate the activation process. The highly electrically charged Ca2+/CaM neutralizes basic regions in the linker domain of CaMKII, facilitating its opening and consequent activation. The emerging picture of CaMKII’s behavior highlights the preponderance of electrostatic interactions, which are modulated by the presence of Ca2+/CaM and the phosphorylation of key sites.
中文翻译:

物理相互作用驱动钙/钙调蛋白依赖性蛋白激酶II的激活/抑制
CaMKII是一种蛋白激酶,其功能受钙/钙调蛋白复合物(Ca 2+ / CaM)结合的调节。它是长期增强过程的主要参与者,在该过程中,它充当分子开关,在受抑制构象和活性构象之间振荡。据认为,用于转换的机制是由Ca 2+ / CaM结合引发的,其允许CaMKII的亚基被邻近的激酶反磷酸化,从而导致系统的活性状态。将全原子和粗粒度MD模拟与自由能计算相结合,使我们揭示了Ca 2+ / CaM对CaMKII施加的静电力的相互作用,从而启动了活化过程。高电荷的Ca 2+/ CaM中和CaMKII接头结构域中的基本区域,从而促进其开放和随后的激活。CaMKII行为的新发现突出了静电相互作用的优势,这些相互作用是通过Ca 2+ / CaM的存在和关键位点的磷酸化来调节的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号