Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-02-27 , DOI: 10.1016/j.cej.2021.129120 Sreetama Ghosh , Joby Sebastian , Louise Olsson , Derek Creaser
|
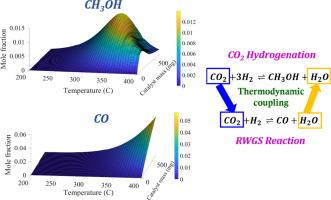
Catalytic hydrogenation of CO2 to methanol has gained considerable interest for its significant role in CO2 utilization using heterogeneous catalysts. This study is the first to propose a kinetic model based on Langmuir-Hinshelwood-Hougen-Watson (LHHW) mechanism for CO2 hydrogenation to methanol over a highly effective indium oxide (In2O3) catalyst. The work focuses on different reaction conditions mainly revolving around the variation of operating temperature, total reactor pressure, H2/CO2 molar feed ratio and weight hourly space velocity (WHSV) of the system. The experimental data were modeled using a competitive single-site kinetic model based on LHHW rate equations. A parameter optimization procedure was undertaken to determine the kinetic parameters of the developed rate equations. The model predicts that when the methanol synthesis reaction becomes equilibrium limited, the progress of the RWGS reaction forces the methanol yield to decrease due to the reversal of the methanol synthesis reaction. A mixture of CO2 and H2 has been used as the reactor feed in all the cases. Significantly w.r.t. the CO2 partial pressure, the reaction rate for methanol synthesis initially increased and then slightly decreased indicating a varying order. The single-site model accurately predicted the trends in the experimental data which would enable the development of reliable reactor and process designs.
中文翻译:

In 2 O 3催化剂由CO 2加氢合成甲醇的实验和动力学模型研究
CO 2催化加氢成甲醇已引起广泛关注,因为它在使用多相催化剂的CO 2利用中起着重要作用。这项研究是首次提出基于Langmuir-Hinshelwood-Hougen-Watson(LHHW)机理的动力学模型,用于在高效氧化铟(In 2 O 3)催化剂上将CO 2加氢成甲醇。这项工作着眼于不同的反应条件,主要围绕操作温度,总反应器压力,H 2 / CO 2的变化而变化。系统的摩尔进料比和重量时空速度(WHSV)。实验数据使用基于LHHW速率方程的竞争性单点动力学模型进行建模。进行了参数优化程序来确定所开发的速率方程的动力学参数。该模型预测,当甲醇合成反应变得平衡受限时,RWGS反应的进行会由于甲醇合成反应的逆转而迫使甲醇产率降低。在所有情况下,已经使用CO 2和H 2的混合物作为反应器进料。显着降低了CO 2分压后,甲醇合成的反应速率开始增加,然后略有下降,表明顺序变化。单站点模型准确地预测了实验数据的趋势,这将有助于开发可靠的反应器和工艺设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号