当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proton selective adsorption on Pt–Ni nano-thorn array electrodes for superior hydrogen evolution activity
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-2-5 , DOI: 10.1039/d1ee00106j Adeela Nairan 1, 2, 3, 4, 5 , Caiwu Liang 1, 2, 3, 4, 5 , Sum-Wai Chiang 1, 2, 3, 4, 5 , Yi Wu 5, 6, 7, 8, 9 , Peichao Zou 1, 2, 3, 4, 5 , Usman Khan 3, 4, 5, 10, 11 , Wendong Liu 5, 6, 7, 8, 9 , Feiyu Kang 1, 2, 3, 4, 5 , Shaojun Guo 5, 12, 13, 14 , Jianbo Wu 5, 6, 7, 8, 9 , Cheng Yang 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2021-2-5 , DOI: 10.1039/d1ee00106j Adeela Nairan 1, 2, 3, 4, 5 , Caiwu Liang 1, 2, 3, 4, 5 , Sum-Wai Chiang 1, 2, 3, 4, 5 , Yi Wu 5, 6, 7, 8, 9 , Peichao Zou 1, 2, 3, 4, 5 , Usman Khan 3, 4, 5, 10, 11 , Wendong Liu 5, 6, 7, 8, 9 , Feiyu Kang 1, 2, 3, 4, 5 , Shaojun Guo 5, 12, 13, 14 , Jianbo Wu 5, 6, 7, 8, 9 , Cheng Yang 1, 2, 3, 4, 5
Affiliation
|
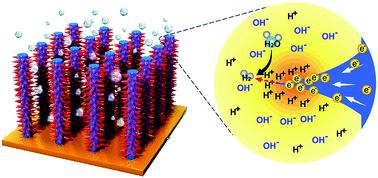
Conventional acidic water electrolysis for large-scale hydrogen production needs to involve a noble metal catalyst for the anode to resist electrochemical oxidation, while alkaline electrolysis can provide better anode protection, but hydrogen ions become a minority species, which leads to sluggish hydrogen evolution reaction (HER) kinetics. Herein, by developing a unique nano-thorn-like Pt–Ni nanowire electrode as a superior HER catalyst, we enable a local “pseudo-acidic” environment near the cathode surface in an alkaline electrolyzer. In such a situation, we observed dramatic enhancement of selective H+ adsorption versus K+, leading to an extremely high HER performance towards real applications, with low overpotentials (ηgeo-surface area) of 23 mV and 71 mV at current densities of 10 mA cm−2 and 200 mA cm−2, respectively. This result is exceptionally better than the state-of-the-art Pt-based catalysts in an alkaline electrolyte at large current densities (≥200 mA cm−2). The simulation result suggests that a strong local electric field around a nano-thorn structure can exponentially increase the diffusion rate of H+ towards the electrode surface as compared with K+, which promotes faster mass transfer and reaction kinetics for the HER in an alkaline medium.
中文翻译:

Pt-Ni纳米刺阵列电极上的质子选择性吸附具有优异的析氢活性
用于大规模制氢的常规酸性水电解需要涉及用于阳极的贵金属催化剂来抵抗电化学氧化,而碱性电解可以提供更好的阳极保护,但是氢离子成为少数物种,导致缓慢的氢生成反应( HER)动力学。在本文中,通过开发独特的纳米刺状Pt-Ni纳米线电极作为优异的HER催化剂,我们在碱性电解槽中的阴极表面附近实现了局部“伪酸性”环境。在这种情况下,我们观察到选择性H +吸附相对于K +显着增强,从而在实际应用中具有极高的HER性能,且超电势较低(η分别在10 mA cm -2和200 mA cm -2的电流密度下分别达到23 mV和71 mV)。在大电流密度(≥200 mA cm -2)下,该结果比碱性电解质中的最新Pt基催化剂好。仿真结果表明,与K +相比,纳米刺结构周围的强局部电场可以指数形式增加H +向电极表面的扩散速率,从而促进了HER在碱性介质中的更快的传质和反应动力学。 。
更新日期:2021-02-26
中文翻译:

Pt-Ni纳米刺阵列电极上的质子选择性吸附具有优异的析氢活性
用于大规模制氢的常规酸性水电解需要涉及用于阳极的贵金属催化剂来抵抗电化学氧化,而碱性电解可以提供更好的阳极保护,但是氢离子成为少数物种,导致缓慢的氢生成反应( HER)动力学。在本文中,通过开发独特的纳米刺状Pt-Ni纳米线电极作为优异的HER催化剂,我们在碱性电解槽中的阴极表面附近实现了局部“伪酸性”环境。在这种情况下,我们观察到选择性H +吸附相对于K +显着增强,从而在实际应用中具有极高的HER性能,且超电势较低(η分别在10 mA cm -2和200 mA cm -2的电流密度下分别达到23 mV和71 mV)。在大电流密度(≥200 mA cm -2)下,该结果比碱性电解质中的最新Pt基催化剂好。仿真结果表明,与K +相比,纳米刺结构周围的强局部电场可以指数形式增加H +向电极表面的扩散速率,从而促进了HER在碱性介质中的更快的传质和反应动力学。 。










































 京公网安备 11010802027423号
京公网安备 11010802027423号