Water Research ( IF 11.4 ) Pub Date : 2021-02-25 , DOI: 10.1016/j.watres.2021.116973 Zhuo-Yu Li , Lu Wang , Yu-Lei Liu , Pei-Nan He , Xin Zhang , Jia Chen , Hai-Teng Gu , Hao-Chen Zhang , Jun Ma
|
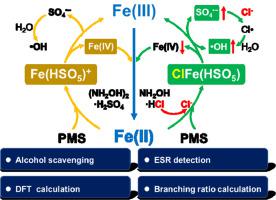
Though hydroxylamine (NH2OH) is effective for accelerating pollutants degradation in Fenton and Fenton-like systems, the effect of anions simultaneously introduced by the hydroxylamine salts have always been ignored. Herein, effect of two commonly used hydroxylamine salts, hydroxylamine hydrochloride (NH2OH·HCl) and hydroxylamine sulfate [(NH2OH)2·H2SO4], for the degradation of dimethyl phthalate (DMP) in peroxymonosulfate (PMS)/Fe(II) system was comparatively investigated. Degradation efficiency of DMP with NH2OH·HCl was 1.6 times of that with same dosages of (NH2OH)2·H2SO4. SO4·−, Fe(IV) and ·OH formed in the PMS/Fe(II)/NH2OH system, but ·OH was the major species for DMP degradation. Addition of Cl− significantly improved the production of ·OH and Cl·, and the exposure dose of ·OH (CT·OH) was more than 10 times that of CTCl· as the concentration of Cl− increased to 1 mM. Calculations based on branching ratios of Cl· and ·OH indicated that the reactions of Cl− with SO4·− and Cl· with H2O were not the only production sources of ·OH in the system. Further experiments with methyl phenyl sulfoxide (PMSO) as the probe indicated that Cl− would facilitate the shift of reactive species from Fe(IV) to radicals (SO4·− or ·OH) in the system. Both hydroxylation and nitration intermediate products were detected in the oxidation of DMP. Cl− promoted the formation of hydroxylation intermediates and reduced the formation of nitration intermediates. This study revealed for the first time that Cl− could shift reactive species from Fe(IV) to radicals in PMS/Fe(II) system, raising attention to the influence of the coexisting anions (especially Cl−) for pollutants oxidation in iron-related oxidation processes.
中文翻译:

氯离子对过氧一硫酸盐/ Fe(II)/ NH 2 OH体系中反应性物种转化的增强作用
尽管羟胺(NH 2 OH)在加速Fenton和类似Fenton的系统中对加速污染物的降解是有效的,但是羟胺盐同时引入的阴离子的作用始终被忽略。本文中,两种常用的羟胺盐,即盐酸羟胺(NH 2 OH · HCl)和硫酸羟胺[[NH 2 OH)2 · H 2 SO 4 ]的作用,用于降解过氧单硫酸盐(PMS)中的邻苯二甲酸二甲酯(DMP)。 / Fe(II)系统进行了比较研究。NH 2 OH · HCl对DMP的降解效率是相同剂量(NH 2 OH)的1.6倍2 · H 2 SO 4。在PMS / Fe(II)/ NH 2 OH系统中形成了SO 4 · −,Fe(IV)和· OH ,但是· OH是DMP降解的主要物质。的Cl加成-显著提高了生产的· OH和Cl ·,和的曝光剂量· OH(CT · OH)为10倍以上的是CT的氯·例如Cl的浓度-增加至1mM。基于Cl ·和·的分支比的计算OH表示,Cl组成的反应-与SO 4 · -和Cl ·用H 2 ö不是唯一的生产源·系统中的OH。用甲基苯基亚砜(PMSO)作为探针进一步的实验表明,氯-将促进反应性物质的选自Fe(IV)的基团,以移位(SO 4 · -或· OH)制得。在DMP的氧化过程中均检测到羟基化和硝化中间产物。氯-促进了羟基化中间体的形成并减少了硝化中间体的形成。这项研究揭示了首次氯-可能的活性物质从铁(IV)在PMS /铁自由基(II)系统转移,提高关注共存阴离子(特别是CI的影响- )中铁污染物氧化相关的氧化过程。









































 京公网安备 11010802027423号
京公网安备 11010802027423号