Biomaterials Advances ( IF 5.5 ) Pub Date : 2021-02-25 , DOI: 10.1016/j.msec.2021.111997 Batiste Clavier , Téo Baptiste , Zuzana Barbieriková , Tomáš Hajdu , Amandine Guiet , Fabien Boucher , Vlasta Brezová , Christine Roques , Gwenaël Corbel
|
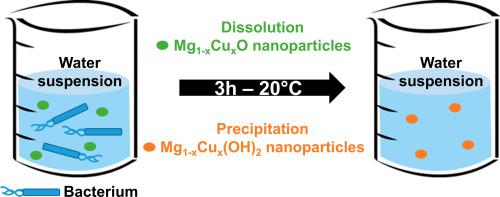
Copper substitution together with nano-structuring are applied with the aim to increase the bactericidal performances of the rocksalt-type MgO oxide. The partial substitution of magnesium ions with Cu2+ has been successfully achieved in both micrometer- and nanometer-sized particles of MgO up to 20 mol% in increments of 5 mol%. Microstructural analyses using the Integral Breadth method revealed that the thermal decomposition of the single source precursor Mg1-xCux(OH)2-2y(CO3)y.zH2O at 400 °C creates numerous defects in 10–20 nm-sized particles of Mg1-xCuxO thus obtained. These defects make the surface of nanoparticles highly reactive towards the sorption of water molecules, to the extent that the cubic cell a parameter in as-prepared Mg1-xCuxO expands by +0.24% as soon as the nanoparticles are exposed to ambient air (60% RH). The hydration of Mg1-xCuxO particles in liquid water is based on a conventional dissolution-precipitation mechanism. Particles of a few microns in size dissolve all the more slowly the higher the copper content and only Mg(OH)2 starts precipitating after 3 h. In contrast, the dissolution of all 10–20 nm-sized Mg1-xCuxO particles is complete over a 3 h period and water suspension only contains 4–12 nm-sized Mg1-xCux(OH)2 particles after 3 h. Thereby, the bactericidal activity reported for water suspension of Mg1-xCuxO nanoparticles depends on the speed at which these nanoparticles dissolve and Mg1-xCux(OH)2 nanoparticles precipitate in the first 3 h. Only 10 mol% of cupric ions in MgO nanoparticles are sufficient to kill both E. coli and S. aureus with a bactericidal kinetics faster and reductions in viability at 3 h (6.5 Log10 and 2.7 Log10, respectively) higher than the conventional antibacterial agent CuO (4.7 Log10 and 2 Log10 under the same conditions). EPR spin trapping study reveals that “hydroxylated” Mg0.9Cu0.1O as well as Mg0.9Cu0.1(OH)2 nanoparticles produce more spin-adducts with highly toxic hydroxyl radicals than their copper-free counterparts. The rapid mass adsorption of Mg0.9Cu0.1(OH)2 nanoparticles onto the cell envelopes following their precipitation together with their ability to produce Reactive Oxygen Species are responsible for the exceptionally high bactericidal activity measured in the course of the hydroxylation of Mg0.9Cu0.1O nanoparticles.
中文翻译:

岩盐型Mg 1-x Cu x O氧化物的纳米和微米级颗粒的水化和杀菌活性
为了提高岩盐型氧化镁的杀菌性能,应用了铜置换和纳米结构化技术。在微米级和纳米级的MgO颗粒中,以20 mol%的增量(5 mol%)成功地实现了镁离子被Cu 2+的部分取代。使用积分宽度法进行的微结构分析表明,单一源前体Mg 1-x Cu x(OH)2-2y(CO 3)y .zH 2 O在400°C时发生热分解会在10–20 nm中产生大量缺陷大小的Mg 1-x Cu x颗粒这样得到的O。这些缺陷使纳米粒子的表面对水分子的吸附具有很高的反应性,以至于一旦纳米粒子暴露于环境中,立方晶胞(Mg 1-x Cu x O中的一个参数)就会扩展+ 0.24%。空气(相对湿度60%)。Mg 1-x Cu x O颗粒在液态水中的水合作用是基于常规的溶解-沉淀机理。铜含量越高,几微米大小的颗粒溶解就越慢,并且3小时后只有Mg(OH)2开始沉淀。相反,所有10–20 nm尺寸的Mg 1-x Cu x的溶解O粒子在3小时内完成,并且水悬浮液在3小时后仅包含4–12 nm大小的Mg 1-x Cu x(OH)2粒子。因此,报告的Mg 1-x Cu x O纳米颗粒水悬浮液的杀菌活性取决于这些纳米颗粒溶解的速度以及前3小时内Mg 1-x Cu x(OH)2纳米颗粒沉淀的速度。MgO纳米颗粒中仅10 mol%的铜离子足以杀死大肠杆菌和金黄色葡萄球菌,并具有更快的杀菌动力学和3小时的活力降低(6.5 Log 10和2.7 Log 10分别高于常规抗菌剂CuO(在相同条件下为4.7 Log 10和2 Log 10)。EPR自旋捕集研究表明,“羟基化” Mg 0.9 Cu 0.1 O以及Mg 0.9 Cu 0.1(OH)2纳米颗粒比无铜对应物产生更多的具有高毒性羟基自由基的自旋加合物。Mg 0.9 Cu 0.1(OH)2的快速质量吸附纳米粒子在沉淀后连同其产生活性氧的能力,进入细胞膜,是在Mg 0.9 Cu 0.1 O纳米粒子羟基化过程中测得的极高杀菌活性的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号