Chemosphere ( IF 8.1 ) Pub Date : 2021-02-26 , DOI: 10.1016/j.chemosphere.2021.130117 Luke T. Townsend , Katherine Morris , Robert Harrison , Bianca Schacherl , Tonya Vitova , Libor Kovarik , Carolyn I. Pearce , J. Frederick W. Mosselmans , Samuel Shaw
|
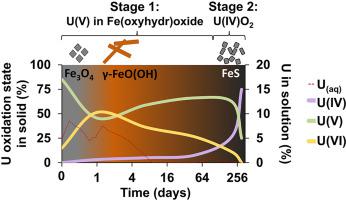
Uranium (U) is a radionuclide of key environmental interest due its abundance by mass within radioactive waste and presence in contaminated land scenarios. Ubiquitously present iron (oxyhydr)oxide mineral phases, such as (nano)magnetite, have been identified as candidates for immobilisation of U via incorporation into the mineral structure. Studies of how biogeochemical processes, such as sulfidation from the presence of sulfate-reducing bacteria, may affect iron (oxyhydr)oxides and impact radionuclide mobility are important in order to underpin geological disposal of radioactive waste and manage radioactively contaminated land. Here, this study utilised a highly controlled abiotic method for sulfidation of U(V) incorporated into nanomagnetite to determine the fate and speciation of U. Upon sulfidation, transient release of U into solution occurred (∼8.6 % total U) for up to 3 days, despite the highly reducing conditions. As the system evolved, lepidocrocite was observed to form over a period of days to weeks. After 10 months, XAS and geochemical data showed all U was partitioned to the solid phase, as both nanoparticulate uraninite (U(IV)O2) and a percentage of retained U(V). Further EXAFS analysis showed incorporation of the residual U(V) fraction into an iron (oxyhydr)oxide mineral phase, likely nanomagnetite or lepidocrocite. Overall, these results provide new insights into the stability of U(V) incorporated iron (oxyhydr)oxides during sulfidation, confirming the longer term retention of U in the solid phase under complex, environmentally relevant conditions.
中文翻译:

掺铀硫化磁铁矿
铀(U)是放射性核素,具有重要的环境意义,因为它在放射性废物中的质量很高,而且在受污染的土地中也存在。普遍存在的(羟基)氧化铁矿物相,例如(纳米)磁铁矿,已被确定为通过掺入矿物结构而固定化U的候选物。为了支持放射性废物的地质处置和管理受放射性污染的土地,对生物地球化学过程(例如因还原硫酸盐的细菌的存在而引起的硫化)如何影响氧化铁(羟基氧化物)和影响放射性核素迁移性的研究非常重要。在这里,这项研究利用高度受控的非生物方法对掺入纳米磁铁矿中的U(V)进行硫化,以确定U的命运和形态。尽管存在高度还原的条件,U仍会在3天内发生短暂释放(约占总U的8.6%)。随着系统的发展,在几天到几周的时间内观察到了纤铁矿的形成。10个月后,XAS和地球化学数据显示,所有U都被划分为固相,因为这两种都是纳米级的尿素(U(IV)O2)和保留的U(V)的百分比。进一步的EXAFS分析表明,残留的U(V)馏分并入了铁(羟基氧化物)矿物相,可能是纳米磁铁矿或纤铁矿。总体而言,这些结果为硫化过程中掺入U(V)的氧化铁(羟基氧化物)的稳定性提供了新的见识,从而证实了U在复杂,与环境有关的条件下在固相中的长期保留。











































 京公网安备 11010802027423号
京公网安备 11010802027423号