当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Vitro Sensitivity Analysis of the Gastrointestinal Dissolution Profile of Weakly Basic Drugs in the Stomach-to-Intestine Fluid Changing System: Explanation for Variable Plasma Exposure after Oral Administration
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-02-25 , DOI: 10.1021/acs.molpharmaceut.0c01207 Toshihide Takagi 1 , Takato Masada 1 , Keiko Minami 1 , Makoto Kataoka 1 , Ken-Ichi Izutsu 2 , Kazuki Matsui 3 , Shinji Yamashita 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-02-25 , DOI: 10.1021/acs.molpharmaceut.0c01207 Toshihide Takagi 1 , Takato Masada 1 , Keiko Minami 1 , Makoto Kataoka 1 , Ken-Ichi Izutsu 2 , Kazuki Matsui 3 , Shinji Yamashita 1
Affiliation
|
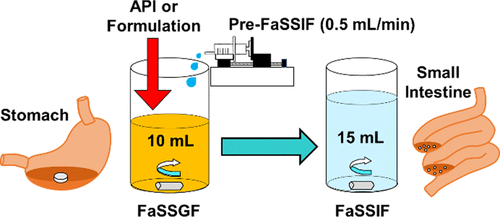
An in vitro methodology for simulating the change in the pH and composition of gastrointestinal fluid associated with the transition of orally administered drugs from the stomach to the small intestine was developed (the stomach-to-intestine fluid changing system (the SIFC system)). This system was applied to in vitro sensitivity analysis on the dissolution of weakly basic drugs, and the obtained results were discussed in relation to the intrasubject variability in the plasma exposure in human bioequivalence (BE) study. Three types of protocols were employed (steep pH change: pH 1.6 FaSSGF → pH 6.5 FaSSIF, gradual pH change: pH 1.6 FaSSGF → pH 6.5 FaSSIF, and high gastric pH: pH 4.0 FaSSGF → pH 6.5 FaSSIF). Regardless of the protocols and the forms of drug applied in active pharmaceutical ingredient powder or formulation, dissolution profiles of pioglitazone after fluid shift were similar and the final concentrations in FaSSIF were approximately equal to the saturation solubility in FaSSIF, supporting its small intrasubject variance in human BE study. In contrast, dissolved concentration of terbinafine in the SIFC system became less than half in the high gastric pH protocol than that in other protocols, suggesting the fluctuation of gastric pH as one of the factors of high intrasubject variance of terbinafine in human. Plasma exposure of telmisartan was highly variable especially at the high dose. Although the dissolution of telmisartan in the SIFC system was greatly improved by formulation, it considerably fluctuated during fluid shift especially at the high dose, which corresponds well to in vivo results.
中文翻译:

胃-肠液体变化系统中弱碱性药物胃肠道溶出曲线的体外敏感性分析:解释口服给药后可变血浆暴露
开发了一种体外方法,用于模拟与口服药物从胃转移到小肠相关的胃肠液的 pH 值和成分变化(胃到肠液变化系统(SIFC 系统))。将该系统应用于弱碱性药物溶出度的体外敏感性分析,并讨论了与人体生物等效性 (BE) 研究中血浆暴露的受试者内部变异性相关的结果。采用了三种类型的方案(pH 值急剧变化:pH 1.6 FaSSGF → pH 6.5 FaSSIF,pH 逐渐变化:pH 1.6 FaSSGF → pH 6.5 FaSSIF,以及高胃酸 pH:pH 4.0 FaSSGF → pH 6.5 FaSSIF)。无论用于活性药物成分粉末或制剂的方案和药物形式如何,流体转移后吡格列酮的溶出曲线相似,并且 FaSSIF 中的最终浓度大约等于 FaSSIF 中的饱和溶解度,支持其在人类 BE 研究中的受试者内部差异很小。相比之下,在高胃 pH 方案中,特比萘芬在 SIFC 系统中的溶解浓度低于其他方案的一半,这表明胃 pH 值的波动是人类特比萘芬高受试者内变异的因素之一。替米沙坦的血浆暴露变化很大,尤其是在高剂量时。虽然替米沙坦在 SIFC 系统中的溶出度通过配方得到了极大的改善,但在流体转移过程中波动很大,尤其是在高剂量时,这与体内结果很好地对应。
更新日期:2021-04-05
中文翻译:

胃-肠液体变化系统中弱碱性药物胃肠道溶出曲线的体外敏感性分析:解释口服给药后可变血浆暴露
开发了一种体外方法,用于模拟与口服药物从胃转移到小肠相关的胃肠液的 pH 值和成分变化(胃到肠液变化系统(SIFC 系统))。将该系统应用于弱碱性药物溶出度的体外敏感性分析,并讨论了与人体生物等效性 (BE) 研究中血浆暴露的受试者内部变异性相关的结果。采用了三种类型的方案(pH 值急剧变化:pH 1.6 FaSSGF → pH 6.5 FaSSIF,pH 逐渐变化:pH 1.6 FaSSGF → pH 6.5 FaSSIF,以及高胃酸 pH:pH 4.0 FaSSGF → pH 6.5 FaSSIF)。无论用于活性药物成分粉末或制剂的方案和药物形式如何,流体转移后吡格列酮的溶出曲线相似,并且 FaSSIF 中的最终浓度大约等于 FaSSIF 中的饱和溶解度,支持其在人类 BE 研究中的受试者内部差异很小。相比之下,在高胃 pH 方案中,特比萘芬在 SIFC 系统中的溶解浓度低于其他方案的一半,这表明胃 pH 值的波动是人类特比萘芬高受试者内变异的因素之一。替米沙坦的血浆暴露变化很大,尤其是在高剂量时。虽然替米沙坦在 SIFC 系统中的溶出度通过配方得到了极大的改善,但在流体转移过程中波动很大,尤其是在高剂量时,这与体内结果很好地对应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号