Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2021-02-25 , DOI: 10.1016/j.psep.2021.02.030 Thanaphat Chukeaw , Worapinit Tiyatha , Kanticha Jaroenpanon , Thongthai Witoon , Paisan Kongkachuichay , Metta Chareonpanich , Kajornsak Faungnawakij , Nevzat Yigit , Günther Rupprechter , Anusorn Seubsai
|
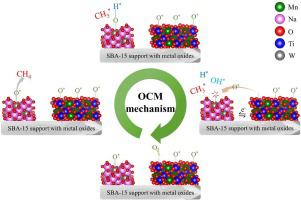
Methane, the main component of natural gas and considered a greenhouse gas, was converted to value-added hydrocarbons (C2+) using synthesized Na2WO4 and MnTiO3 supported on SBA-15 through the oxidative coupling of methane. The MnTiO3 co-impregnated with Na2WO4 on SBA-15 (MnTiO3-NW/SBA-15) was more active than catalysts that were prepared using a simple one-pot impregnation of Mn2+, Ti4+, and Na2WO4 over fumed-SiO2, MCM-41, or SBA-15. Characterizations of the catalysts suggested that the presence of MnTiO3 was more important in the activation of methane relative to Mn2O3. The highest C2+ yield obtained from MnTiO3-NW/SBA-15 was 24.9% with 63.0% C2+ selectivity and 39.4% CH4 conversion at a reaction temperature of 700 °C, a feed ratio of CH4:O2:N2 = 3:1:4, and a space velocity of 18,500 h-1. A time-on-stream test of MnTiO3-NW/SBA-15 over 25 h revealed that the C2+ yield slowly decreased from 24.9% to 20.2% at the end of the 25 h run, because of a decrease in the catalyst’s surface area, a loss of active Na2WO4 from the catalyst, and the destruction of α-cristobalite. Experiments using in-situ diffuse reflectance infrared Fourier transform spectroscopy with mass spectrometry, operated at 475 °C, revealed that the MnTiO3-NW/SBA-15 catalyst enhanced the formation of CH3 and H radicals, while no products or intermediates were found when only SBA-15 was tested.
中文翻译:

MnTiO 3 -Na 2 WO 4 / SBA-15催化剂上甲烷的氧化偶联合成增值烃
甲烷是天然气的主要成分,被认为是一种温室气体,利用甲烷的氧化偶联作用,将合成的Na 2 WO 4和MnTiO 3负载在SBA-15上,将其转化为高附加值的碳氢化合物(C 2+)。所述MnTiO 3共浸渍用Na 2 WO 4上SBA-15(MnTiO 3 -NW / SBA-15)比使用锰的简单一锅浸渍制备的催化剂更具活性2+,钛4+,和Na 2 WO 4在热解法SiO 2,MCM-41或SBA-15上。催化剂的表征表明MnTiO的存在3是相对于甲烷的活化与Mn更重要2 ö 3。从MnTiO 3 -NW / SBA-15获得的最高C 2+产率为24.9%,在700°C的反应温度下,CH 4:O 2的进料比具有63.0%的C 2+选择性和39.4%的CH 4转化率。:N 2 = 3:1:4,并且空速为18,500 h -1。MnTiO 3 -NW / SBA-15经过25小时的运行时间测试显示,在25小时运行结束时,C 2+的收率从24.9%缓慢降低至20.2%,这是由于催化剂的降低所致。表面积,活性钠的损失2 WO 4由催化剂中和,破坏α-方英石。在475°C下使用原位漫反射红外傅里叶变换质谱联用质谱进行的实验表明,MnTiO 3 -NW / SBA-15催化剂可增强CH 3和H自由基的形成,而未发现任何产物或中间体仅测试了SBA-15。











































 京公网安备 11010802027423号
京公网安备 11010802027423号