Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2021-02-25 , DOI: 10.1016/j.fluid.2021.112987 Alexander Keller , Irenäus Wlokas , Maximilian Kohns , Hans Hasse
|
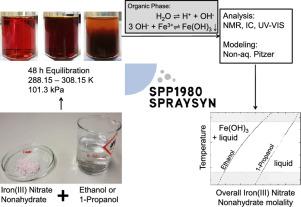
Solutions of iron(III) nitrate nonahydrate (INN) in ethanol and 1-propanol are interesting starting materials for producing iron oxide nanoparticles by spray flame synthesis. For the design of these processes, it is important to have information on solid-liquid equilibria (SLE) in these solutions. As corresponding data were not available in the literature, we have studied the SLE of solutions of INN in ethanol and 1-propanol experimentally at temperatures between 288.15 and 308.15 K at 101.3 kPa. Unexpected phenomena occur in these solutions: one would expect an increasing amount of precipitate upon increasing the concentration of the salt INN, but the reverse behavior is observed for a wide range of states. This is caused by chemical reactions in the solutions: not INN, but iron(III) hydroxide (IH) precipitates from the solution, leading also to a strong acidity of the mixture. These chemical effects are taken into account in a physico-chemical model that was developed for describing the SLE in the studied systems. In that model, the physical non-ideality is described with the extended Pitzer model.
中文翻译:

硝酸亚铁三水合物与乙醇或1-丙醇混合物中的固液平衡
硝酸铁九水合物(INN)在乙醇和1-丙醇中的溶液是通过喷雾火焰合成生产氧化铁纳米颗粒的有趣起始原料。对于这些过程的设计,在这些解决方案中获得固液平衡(SLE)信息非常重要。由于文献中没有相应的数据,因此我们在288.15至308.15 K之间,温度为101.3 kPa的条件下,对INN的乙醇和1-丙醇溶液的SLE进行了研究。这些解决方案中发生了无法预料的现象:人们会期望随着盐INN浓度的增加,沉淀物的数量也会增加,但是在很宽的状态下观察到相反的现象。这是由溶液中的化学反应引起的:不是INN,而是氢氧化铁(III)从溶液中沉淀出来,也导致混合物的强酸度。在描述所研究系统中SLE的物理化学模型中考虑了这些化学作用。在该模型中,使用扩展的Pitzer模型描述了物理上的非理想状态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号