Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of folic acid and protonation of folate in NaCl at different concentrations, even in physiological solution
Analyst ( IF 3.6 ) Pub Date : 2021-2-11 , DOI: 10.1039/d1an00013f Emilio Bottari 1, 2, 3, 4 , Antonietta D'Ambrosio 1, 2, 3, 4 , Gaetano De Tommaso 4, 5, 6, 7 , Maria Rosa Festa 1, 2, 3, 4 , Mauro Iuliano 4, 5, 6, 7 , Martina Meschino 1, 2, 3, 4
Analyst ( IF 3.6 ) Pub Date : 2021-2-11 , DOI: 10.1039/d1an00013f Emilio Bottari 1, 2, 3, 4 , Antonietta D'Ambrosio 1, 2, 3, 4 , Gaetano De Tommaso 4, 5, 6, 7 , Maria Rosa Festa 1, 2, 3, 4 , Mauro Iuliano 4, 5, 6, 7 , Martina Meschino 1, 2, 3, 4
Affiliation
|
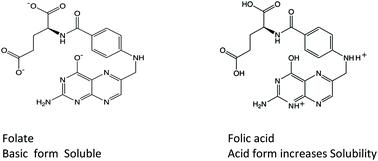
The solubility of folic acid was determined at 25 °C in 1.00 mol dm−3 and in 0.15 mol dm−3 NaCl (physiological solution) spectrophotometrically by measuring the absorbance of saturated solution at different hydrogen ion concentrations. Five protonation constants of folate were determined both from the dependence of the solubility on the hydrogen ion concentration as well as from potentiometric titrations carried out in the presence of solid folic acid and in alkaline solution, in which folate is relatively soluble. Corresponding to the protonation constants, nuclear magnetic resonance and florescence spectra were also obtained at different hydrogen ion concentrations to determine the protonation positions in acid, neutral and alkaline solutions. An approach through circular dichroism was also applied to study the eventual polymerization of folate in alkaline solution.
中文翻译:

在不同浓度的NaCl中,即使在生理溶液中,叶酸的溶解度和叶酸的质子化
在25°C下于1.00 mol dm -3和0.15 mol dm -3中测定叶酸的溶解度通过在不同的氢离子浓度下测量饱和溶液的吸光度,用分光光度法测定NaCl(生理溶液)。从溶解度对氢离子浓度的依赖性以及在固体叶酸存在下和在碱性溶液中进行的电位滴定确定了叶酸的五个质子化常数,叶酸相对易溶。对应于质子化常数,还获得了在不同氢离子浓度下的核磁共振和荧光光谱,以确定在酸性,中性和碱性溶液中的质子化位置。还通过圆二色性方法研究了叶酸在碱性溶液中的最终聚合。
更新日期:2021-02-24
中文翻译:

在不同浓度的NaCl中,即使在生理溶液中,叶酸的溶解度和叶酸的质子化
在25°C下于1.00 mol dm -3和0.15 mol dm -3中测定叶酸的溶解度通过在不同的氢离子浓度下测量饱和溶液的吸光度,用分光光度法测定NaCl(生理溶液)。从溶解度对氢离子浓度的依赖性以及在固体叶酸存在下和在碱性溶液中进行的电位滴定确定了叶酸的五个质子化常数,叶酸相对易溶。对应于质子化常数,还获得了在不同氢离子浓度下的核磁共振和荧光光谱,以确定在酸性,中性和碱性溶液中的质子化位置。还通过圆二色性方法研究了叶酸在碱性溶液中的最终聚合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号