当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Catalytic Mechanism of Domains CD1 and CD2 in Histone Deacetylase 6 from Quantum Calculations
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-23 , DOI: 10.1021/acscatal.0c04729 Mario Prejanò 1 , Pietro Vidossich 2 , Nino Russo 1 , Marco De Vivo 2 , Tiziana Marino 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-02-23 , DOI: 10.1021/acscatal.0c04729 Mario Prejanò 1 , Pietro Vidossich 2 , Nino Russo 1 , Marco De Vivo 2 , Tiziana Marino 1
Affiliation
|
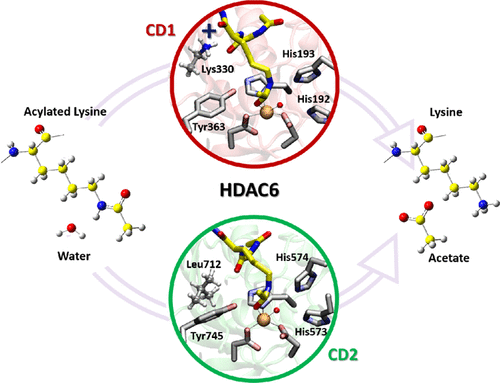
The histone deacetylase 6 enzyme, HDAC6, is a metalloprotein with multiple pathophysiological roles and, as such, is the subject of intense research and drug discovery efforts. Different from the HDAC family, HDAC6 is constituted by a heterodimeric structure with two different catalytic domains, namely CD1 and CD2. Intriguingly, the specific biological relevance of each domain is not fully understood yet. However, a wealth of structural and biochemical data collected on HDAC, and in particular more recently on HDAC6, has revealed that the active sites in both CD1 and CD2 conserve a similar structural architecture. In the present work, we have performed a detailed investigation on the mechanistic aspects of catalysis of HDAC6, comparatively analyzing the enzymatic reaction mechanism in CD1 and CD2. Using density functional theory-based computations, we found that the rate-limiting step of the reaction is the nucleophilic attack of the catalytic water for peptide bond hydrolysis in both domains. Remarkably, the transition state for such a step presents a penta-coordinated metal center. In this regard, we have further validated the crucial contribution to catalysis of Tyr residues (Tyr363 in CD1 and Tyr745 in CD2) for the activation of the substrate, in line with kinetics measures. Importantly, we have quantified the effect on catalysis by the positively charged Lys330 residue in CD1. An analogous contribution is missing in CD2. Our calculations also evidence the contribution of distal residues from the reaction center, such as Gly201/582 and Phe202/583. Taken together, our quantum calculations dissect the catalytic mechanism in HDAC6. These findings may help future experimental studies and drug design efforts for selective HDAC6 targeting.
中文翻译:

从量子计算洞察组蛋白脱乙酰基酶6中的域CD1和CD2的催化机理。
组蛋白脱乙酰基酶6酶HDAC6是一种具有多种病理生理作用的金属蛋白,因此,它是深入研究和药物发现努力的主题。与HDAC家族不同,HDAC6由具有两个不同催化域(即CD1和CD2)的异二聚体结构构成。有趣的是,每个域的具体生物学相关性尚未完全理解。但是,从HDAC,尤其是最近在HDAC6上收集到的大量结构和生化数据表明,CD1和CD2中的活性位点均保留相似的结构。在目前的工作中,我们对HDAC6催化机理进行了详细的研究,比较了CD1和CD2中的酶促反应机理。使用基于密度泛函理论的计算,我们发现该反应的限速步骤是在两个域中用于肽键水解的催化水的亲核攻击。显着地,用于该步骤的过渡状态呈现五配位的金属中心。在这方面,我们已根据动力学方法进一步验证了催化Tyr残基(CD1中的Tyr363和CD2中的Tyr745)对底物活化的关键作用。重要的是,我们已经量化了CD1中带正电荷的Lys330残基对催化的影响。CD2中缺少类似的贡献。我们的计算还证明了来自反应中心的远端残基的贡献,例如Gly201 / 582和Phe202 / 583。综上所述,我们的量子计算剖析了HDAC6中的催化机理。
更新日期:2021-03-05
中文翻译:

从量子计算洞察组蛋白脱乙酰基酶6中的域CD1和CD2的催化机理。
组蛋白脱乙酰基酶6酶HDAC6是一种具有多种病理生理作用的金属蛋白,因此,它是深入研究和药物发现努力的主题。与HDAC家族不同,HDAC6由具有两个不同催化域(即CD1和CD2)的异二聚体结构构成。有趣的是,每个域的具体生物学相关性尚未完全理解。但是,从HDAC,尤其是最近在HDAC6上收集到的大量结构和生化数据表明,CD1和CD2中的活性位点均保留相似的结构。在目前的工作中,我们对HDAC6催化机理进行了详细的研究,比较了CD1和CD2中的酶促反应机理。使用基于密度泛函理论的计算,我们发现该反应的限速步骤是在两个域中用于肽键水解的催化水的亲核攻击。显着地,用于该步骤的过渡状态呈现五配位的金属中心。在这方面,我们已根据动力学方法进一步验证了催化Tyr残基(CD1中的Tyr363和CD2中的Tyr745)对底物活化的关键作用。重要的是,我们已经量化了CD1中带正电荷的Lys330残基对催化的影响。CD2中缺少类似的贡献。我们的计算还证明了来自反应中心的远端残基的贡献,例如Gly201 / 582和Phe202 / 583。综上所述,我们的量子计算剖析了HDAC6中的催化机理。










































 京公网安备 11010802027423号
京公网安备 11010802027423号