Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PRMT5 Enables Robust STAT3 Activation via Arginine Symmetric Dimethylation of SMAD7
Advanced Science ( IF 14.3 ) Pub Date : 2021-02-24 , DOI: 10.1002/advs.202003047 Congcong Cai 1, 2 , Shuchen Gu 1, 2 , Yi Yu 1, 2 , Yezhang Zhu 1 , HanChenxi Zhang 1 , Bo Yuan 1 , Li Shen 1 , Bing Yang 1 , Xin-Hua Feng 1, 2, 3
Advanced Science ( IF 14.3 ) Pub Date : 2021-02-24 , DOI: 10.1002/advs.202003047 Congcong Cai 1, 2 , Shuchen Gu 1, 2 , Yi Yu 1, 2 , Yezhang Zhu 1 , HanChenxi Zhang 1 , Bo Yuan 1 , Li Shen 1 , Bing Yang 1 , Xin-Hua Feng 1, 2, 3
Affiliation
|
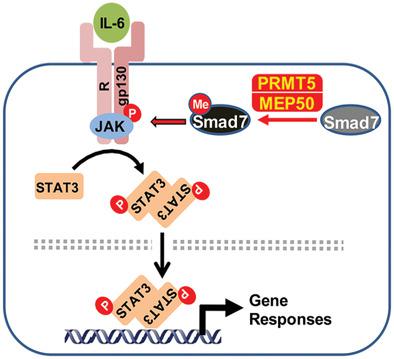
Protein arginine methyltransferase 5 (PRMT5) is the type II arginine methyltransferase that catalyzes the mono- and symmetrical dimethylation of protein substrates at the arginine residues. Emerging evidence reveals that PRMT5 is involved in the regulation of tumor cell proliferation and cancer development. However, the exact role of PRMT5 in human lung cancer cell proliferation and the underlying molecular mechanism remain largely elusive. Here, it is shown that PRMT5 promotes lung cancer cell proliferation through the Smad7-STAT3 axis. Depletion or inhibition of PRMT5 dramatically dampens STAT3 activation and thus suppresses the proliferation of human lung cancer cells. Furthermore, depletion of Smad7 blocks PRMT5-mediated STAT3 activation. Mechanistically, PRMT5 binds to and methylates Smad7 on Arg-57, enhances Smad7 binding to IL-6 co-receptor gp130, and consequently ensures robust STAT3 activation. The findings position PRMT5 as a critical regulator of STAT3 activation, and suggest it as a potential therapeutic target for the treatment of human lung cancer.
中文翻译:

PRMT5 通过 SMAD7 的精氨酸对称二甲基化实现 STAT3 的稳健激活
蛋白质精氨酸甲基转移酶 5 (PRMT5) 是 II 型精氨酸甲基转移酶,可催化蛋白质底物在精氨酸残基处的单对称二甲基化。新的证据表明 PRMT5 参与肿瘤细胞增殖和癌症发展的调节。然而,PRMT5 在人肺癌细胞增殖中的确切作用及其潜在的分子机制仍然很大程度上难以捉摸。在此,显示 PRMT5 通过 Smad7-STAT3 轴促进肺癌细胞增殖。 PRMT5 的缺失或抑制可显着抑制 STAT3 的激活,从而抑制人肺癌细胞的增殖。此外,Smad7 的缺失会阻断 PRMT5 介导的 STAT3 激活。从机制上讲,PRMT5 与 Arg-57 上的 Smad7 结合并甲基化,增强 Smad7 与 IL-6 共受体 gp130 的结合,从而确保 STAT3 的稳健激活。这些发现将 PRMT5 定位为 STAT3 激活的关键调节因子,并表明它是治疗人类肺癌的潜在治疗靶点。
更新日期:2021-02-24
中文翻译:

PRMT5 通过 SMAD7 的精氨酸对称二甲基化实现 STAT3 的稳健激活
蛋白质精氨酸甲基转移酶 5 (PRMT5) 是 II 型精氨酸甲基转移酶,可催化蛋白质底物在精氨酸残基处的单对称二甲基化。新的证据表明 PRMT5 参与肿瘤细胞增殖和癌症发展的调节。然而,PRMT5 在人肺癌细胞增殖中的确切作用及其潜在的分子机制仍然很大程度上难以捉摸。在此,显示 PRMT5 通过 Smad7-STAT3 轴促进肺癌细胞增殖。 PRMT5 的缺失或抑制可显着抑制 STAT3 的激活,从而抑制人肺癌细胞的增殖。此外,Smad7 的缺失会阻断 PRMT5 介导的 STAT3 激活。从机制上讲,PRMT5 与 Arg-57 上的 Smad7 结合并甲基化,增强 Smad7 与 IL-6 共受体 gp130 的结合,从而确保 STAT3 的稳健激活。这些发现将 PRMT5 定位为 STAT3 激活的关键调节因子,并表明它是治疗人类肺癌的潜在治疗靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号