Forensic Chemistry ( IF 2.6 ) Pub Date : 2021-02-24 , DOI: 10.1016/j.forc.2021.100326 Younis Abiedalla , Ahmad J. Almalki , Jack DeRuiter , C. Randall Clark
|
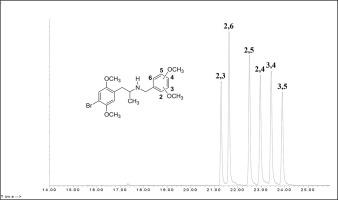
The N-(methoxybenzyl- and dimethoxybenzyl)-4-bromo-2,5-dimethoxyphenylisopropylamines are a likely category of novel psychoactive substances based on a combination of structural features for the amphetamine and the NBOMe drugs. The nine compounds in this study were synthesized from readily available precursor materials via a common synthetic pathway. The gas chromatographic separations of the monomethoxybenzyl- and dimethoxybenzyl subseries of regioisomers were achieved on a midpolarity capillary column and the elution order appears related to aromatic ring substituent crowding.
The significant ions in the EI-MS spectra consist of the iminium cation and other fragments originating from the nonbrominated benzylamine portion of the molecules. These observations are consistent with those of the traditional unbranched phenethyl NBOMe analogues. The mass of the major fragments varies based on the number of methoxy groups of the benzyl aromatic ring. Within each subseries of monomethoxy and dimethoxy analogues, the mass spectra are remarkably similar with slight variations in relative abundance in most cases. Minor ions containing bromine occur for the 4-bromo-2,5-dimethoxybenzyl cation and its product ion resulting from the rearrangement loss of the 2-methoxy group in the form of formaldehyde. The vapor phase infrared spectra generated in GC–IR experiments provide data for the determination of the position of substitution of the methoxy groups on the benzyl aromatic ring in both the monomethoxy- and dimethoxybenzyl subseries.
中文翻译:

取代的N-苄基4-溴-2,5-二甲氧基苯基异丙基胺的GC–MS和GC–IR分析
所述ñ - (甲氧基苄基和二甲氧基苄基)-4-溴-2,5- dimethoxyphenylisopropylamines是基于为安非他明和NBOMe药物的结构特征的组合的新的精神活性物质的可能的类别。本研究中的九种化合物是通过通用的合成途径从易得的前体材料中合成的。在中极性毛细管柱上进行了区域异构体的单甲氧基苄基和二甲氧基苄基亚系列的气相色谱分离,洗脱顺序似乎与芳香环取代基的拥挤有关。
EI-MS光谱中的重要离子由亚胺阳离子和源自分子非溴化苄胺部分的其他片段组成。这些观察结果与传统的非支链苯乙基NBOMe类似物的观察结果一致。主要片段的质量基于苄基芳族环的甲氧基的数量而变化。在单甲氧基和二甲氧基类似物的每个亚系列中,质谱在大多数情况下都非常相似,相对丰度略有变化。对于4-溴-2,5-二甲氧基苄基阳离子存在含溴的次离子,其产物离子是由2-甲氧基以甲醛形式的重排损失产生的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号