Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2021-02-24 , DOI: 10.1016/j.jallcom.2021.159311 Shuo Geng , Yarong Huang , Akhmat Fauzi , Yongsheng Yu , Yequn Liu , Weiwei Yang
|
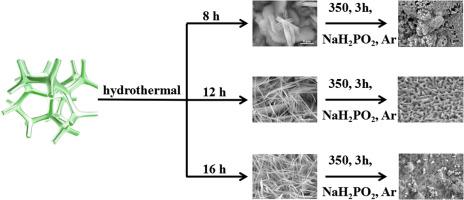
The synthesis of highly efficient non-noble bifunctional electrocatalysts for both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in the same electrolyte is important for the industrial application of electrocatalytic water splitting. Herein, non-noble bifunctional electrocatalyst Ni0.67Co0.33(PO3)2 nets with optimized morphology were successfully synthesized. Owing to the micropores with the accelerated electrolyte and gases transfer and nanopores architecture with more exposed active edge sites, the self-supported Ni0.67Co0.33(PO3)2-12 h electrode exhibits a superior water splitting performance. Impressively, the Ni0.67Co0.33(PO3)2-12 h electrode only needs a cell voltage of 1.46 V to achieve 10 mA/cm2 and shows over 22 h stability at 10, 50 and 100 mA/cm2, respectively. This present work suggests a unique and earth abundant material synthesis method for water electrolysis, which has broad prospects for practical water splitting application.
中文翻译:

NiCo层状双氢氧化物衍生的Ni 0.67 Co 0.33(PO 3)2作为稳定和高效的整体水分解电催化剂
在同一个电解质中合成用于氢气释放反应(HER)和氧气释放反应(OER)的高效非贵金属双功能电催化剂对于电催化水分解的工业应用非常重要。在此,成功地合成了具有优化形态的非贵金属双功能电催化剂Ni 0.67 Co 0.33(PO 3)2。由于具有加速的电解质和气体传输的微孔以及具有更多暴露的活性边缘位点的纳米孔结构,自支撑的Ni 0.67 Co 0.33(PO 3)2-12小时电极表现出优异的水分解性能。令人印象深刻的是,Ni 0.67 Co 0.33(PO 3)2 -12 h电极仅需要1.46 V的电池电压即可达到10 mA / cm 2,并分别在10、50和100 mA / cm 2下显示超过22 h的稳定性。这项工作提出了一种独特的,富含土壤的水电解材料合成方法,在实际的水分解应用中具有广阔的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号