Chemosphere ( IF 8.1 ) Pub Date : 2021-02-24 , DOI: 10.1016/j.chemosphere.2021.129848 Qicheng Qiao , Seema Singh , Shang-Lien Lo , Jierong Jin , Yong chang Yu , Lizhang Wang
|
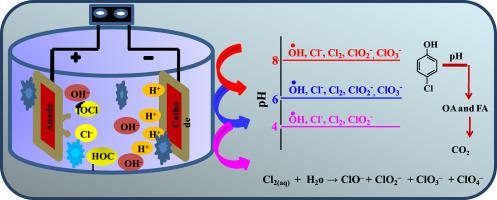
The aim of present study is increasing the degradation and mineralization of 4-chlorophenol (4-CP) during electrochemical oxidation with Ti/RuO2 anodes. Innovatively, the evolution of chlorine-related species and the formations of various inorganic ions were investigated by electrolytic analysis in order to set up whether the formation and consumption of these byproducts associated with either chemical or electrochemical reactions. The effect of operating parameters such as current density, solution pH, treatment time, and electrolyte concentration has been studied. The formation of Cl2, chlorite (ClO2−), and chlorate (ClO3−) were detected by adding the known concentration of Cl− ions at different pH and current densities. Concentration trends of active chloro-species indicate that the degradation of 4-CP and chemical oxygen demand (COD) removal was formed maximum at pH 6 and j of 225.2 Am−2 in presence of 0.0085 M NaCl. Thus, the 4-CP degradation mainly depends on the radicals and active chlorine formation and a mineralization mechanism was proposed based on intermediates byproducts formation such as catechol, hydroquinone, 1, 4-benzoquinone, and organic acids identify by using the GC-MS and HPLC analysis at the optimum treatment condition. Total organic carbon (TOC) at different pH and current density, mass balance analysis of carbon and inorganic species formation were determined at the optimum treatment conditions of 4-CP. The degradation kinetic of 4-CP was followed the pseudo-first order kinetic model during the each parameters optimization. Specific energy consumption and current efficiency were also used to identify the technical feasibility of the process.
中文翻译:

电流密度和pH值对用于快速矿化对位取代苯酚的电化学生成的活性氯物质的影响
本研究的目的是增加在用Ti / RuO 2阳极进行电化学氧化过程中4-氯苯酚(4-CP)的降解和矿化作用。创新地,通过电解分析研究了与氯有关的物种的演变以及各种无机离子的形成,以便确定这些副产物的形成和消耗是否与化学或电化学反应有关。已经研究了诸如电流密度,溶液pH,处理时间和电解质浓度等操作参数的影响。氯的形成2,绿泥石(CLO 2 - ,和氯酸盐(CLO)3 - ),通过加入Cl组成的已知浓度检测-离子在不同的pH和电流密度下。活性氯物种的浓度趋势表明,在pH 6和j为225.2 Am -2时,最大程度地形成了4-CP的降解和化学需氧量(COD)的去除。在0.0085 M NaCl存在下。因此,4-CP的降解主要取决于自由基和活性氯的形成,并基于中间产物副产物的形成提出了矿化机理,所述中间产物如邻苯二酚,对苯二酚,1,4-苯醌和有机酸通过GC-MS鉴定。在最佳处理条件下进行HPLC分析。在4-CP的最佳处理条件下,确定了在不同pH和电流密度下的总有机碳(TOC),碳和无机物质形成的质量平衡分析。在每个参数优化过程中,遵循伪一级动力学模型对4-CP进行降解。还使用了特定的能耗和电流效率来确定该工艺的技术可行性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号