Chemosphere ( IF 8.8 ) Pub Date : 2021-02-23 , DOI: 10.1016/j.chemosphere.2021.130087 Farhad Ahmadijokani , Shima Tajahmadi , Mahdi Heidarian Haris , Addie Bahi , Mashallah Rezakazemi , Hossein Molavi , Frank Ko , Mohammad Arjmand
|
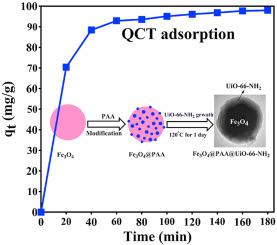
In the present study, a magnetic core-shell metal-organic framework (Fe3O4@PAA@UiO-66-NH2) nanocomposite was synthesized by a facile step-by-step self-assembly technique and used for selective adsorption of the anti-cancer Quercetin (QCT) drug. The synthesized nanocomposite was well characterized using FTIR, XRD, BET, FESEM, and TEM techniques. The adsorption kinetics and isotherms of the magnetic nanocomposites for QCT were investigated in detail at different initial concentrations and temperatures. It was found that the experimental adsorption kinetic and isotherm data were precisely explained by the pseudo-second-order kinetic and Langmuir isotherm models. Moreover, the selective adsorption ability of the synthesized nanocomposite against various drugs in the single, binary, and ternary solutions containing QCT, Curcumin (CUR), and Methotrexate (MTX) drugs was also studied. The synthesized adsorbent showed good adsorption selectivity for QCT against CUR and MTX. The adsorption mechanism of QCT on the nanocomposite might be related to the hydrogen bonding and hydrophobic-hydrophobic interactions via π-π stacking interactions between the benzene ring skeleton of QCT and the aromatic structure of the adsorbent nanoparticles. The regeneration and reusability studies demonstrated that the developed adsorbent sustained good structural stability and adequate adsorption capacity for QCT after ten consecutive adsorption-desorption cycles.
中文翻译:

Fe3O4 @ PAA @ UiO-66-NH2磁性纳米复合材料对槲皮素的选择性吸附
在本研究中,磁性核-壳金属-有机骨架(Fe 3 O 4 @ PAA @ UiO-66-NH 2纳米复合材料是通过简便的逐步自组装技术合成的,并用于抗癌槲皮素(QCT)药物的选择性吸附。使用FTIR,XRD,BET,FESEM和TEM技术对合成的纳米复合材料进行了很好的表征。在不同的初始浓度和温度下,详细研究了磁性纳米复合材料对QCT的吸附动力学和等温线。发现通过准二级动力学和Langmuir等温线模型可以精确地解释实验的吸附动力学和等温线数据。此外,还研究了合成的纳米复合材料在含有QCT,姜黄素(CUR)和甲氨蝶呤(MTX)药物的单一,二元和三元溶液中对各种药物的选择性吸附能力。合成的吸附剂对QCT对CUR和MTX表现出良好的吸附选择性。QCT在纳米复合材料上的吸附机理可能与QCT的苯环骨架与吸附剂纳米颗粒的芳族结构之间的π-π堆积相互作用与氢键键合和疏水-疏水相互作用有关。再生和可重复使用性研究表明,经过十次连续的吸附-解吸循环后,所开发的吸附剂对QCT保持了良好的结构稳定性和足够的吸附能力。QCT在纳米复合材料上的吸附机理可能与QCT的苯环骨架与吸附剂纳米颗粒的芳族结构之间的π-π堆积相互作用与氢键键合和疏水-疏水相互作用有关。再生和可重复使用性研究表明,经过十次连续的吸附-解吸循环后,所开发的吸附剂对QCT保持了良好的结构稳定性和足够的吸附能力。QCT在纳米复合材料上的吸附机理可能与QCT的苯环骨架与吸附剂纳米颗粒的芳族结构之间的π-π堆积相互作用与氢键键合和疏水-疏水相互作用有关。再生和可重复使用性研究表明,经过十次连续的吸附-解吸循环后,所开发的吸附剂对QCT保持了良好的结构稳定性和足够的吸附能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号