当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How Do Electrostatic Perturbations of the Protein Affect the Bifurcation Pathways of Substrate Hydroxylation versus Desaturation in the Nonheme Iron-Dependent Viomycin Biosynthesis Enzyme?
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2021-02-23 , DOI: 10.1021/acs.jpca.1c00141 Hafiz Saqib Ali 1, 2 , Richard H Henchman 1, 2 , Jim Warwicker 1, 3 , Sam P de Visser 1, 4
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2021-02-23 , DOI: 10.1021/acs.jpca.1c00141 Hafiz Saqib Ali 1, 2 , Richard H Henchman 1, 2 , Jim Warwicker 1, 3 , Sam P de Visser 1, 4
Affiliation
|
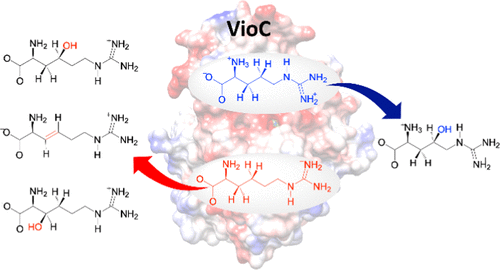
The viomycin biosynthesis enzyme VioC is a nonheme iron and α-ketoglutarate-dependent dioxygenase involved in the selective hydroxylation of l-arginine at the C3-position for antibiotics biosynthesis. Interestingly, experimental studies showed that using the substrate analogue, namely, l-homo-arginine, a mixture of products was obtained originating from C3-hydroxylation, C4-hydroxylation, and C3–C4-desaturation. To understand how the addition of one CH2 group to a substrate can lead to such a dramatic change in selectivity and activity, we decided to perform a computational study using quantum mechanical (QM) cluster models. We set up a large active-site cluster model of 245 atoms that includes the oxidant with its first- and second-coordination sphere influences as well as the substrate binding pocket. The model was validated against experimental work from the literature on related enzymes and previous computational studies. Thereafter, possible pathways leading to products and byproducts were investigated for a model containing l-Arg and one for l-homo-Arg as substrate. The calculated free energies of activation predict product distributions that match the experimental observation and give a low-energy C3-hydroxylation pathway for l-Arg, while for l-homo-Arg, several barriers are found to be close in energy leading to a mixture of products. We then analyzed the origins of the differences in product distributions using thermochemical, valence bond, and electrostatic models. Our studies show that the C3–H and C4–H bond strengths of l-Arg and l-homo-Arg are similar; however, external perturbations from an induced electric field of the protein affect the relative C–H bond strengths of l-Arg dramatically and make the C3–H bond the weakest and guide the reaction to a selective C3-hydroxylation channel. Therefore, the charge distribution in the protein and the induced electric dipole field of the active site of VioC guides the l-Arg substrate activation to C3-hydroxylation and disfavors the C4-hydroxylation pathway, while this does not occur for l-homo-Arg. Tight substrate positioning and electrostatic perturbations from the second-coordination sphere residues in VioC also result in a slower overall reaction for l-Arg; however, they enable a high substrate selectivity. Our studies highlight the importance of the second-coordination sphere in proteins that position the substrate and oxidant, perturb charge distributions, and enable substrate selectivity.
中文翻译:

蛋白质的静电扰动如何影响非血红素依赖性铁霉素丝裂霉素生物合成酶中底物羟化与去饱和的分叉途径?
紫霉素生物合成酶VioC是非血红素铁和α-酮戊二酸依赖性双加氧酶,参与抗生素生物合成的C 3位上1-精氨酸的选择性羟基化。有趣的是,实验研究表明,使用底物类似物,即1 -homo-精氨酸,获得了源自C 3-羟基化,C 4-羟基化和C 3 -C 4-去饱和的产物混合物。了解如何添加一个CH 2基团与底物的结合可能导致选择性和活性的急剧变化,我们决定使用量子力学(QM)簇模型进行计算研究。我们建立了一个包含245个原子的大型活性位点簇模型,其中包括氧化剂及其第一,第二配位球的影响以及底物的结合口袋。该模型已针对相关酶文献和先前的计算研究的实验工作进行了验证。此后,针对含有1 -Arg的模型和以1 -homo-Arg作为底物的模型研究了导致产物和副产物的可能途径。计算出的活化自由能预测与实验观察结果相符的产物分布,并给出低能的C 3。羟基化途径升-精氨酸,而升-homo精氨酸,一些障碍被发现是接近能源导致的产物的混合物。然后,我们使用热化学,价键和静电模型分析了产品分布差异的起因。我们的研究表明,l -Arg和l -homo-Arg的C 3 -H和C 4 -H键强度相似;然而,蛋白质感应电场的外部扰动会显着影响l -Arg的相对C–H键强度,并使C 3 –H键最弱,并将反应引导至选择性C 3-羟基化通道。因此,蛋白质的电荷分布和VioC活性位点的感应电偶极子场会引导1- Arg底物活化至C 3-羟基化,并不利于C 4-羟基化途径,而对于l -homo不会发生-Arg 紧基板定位和静电扰动从VIOC第二配位球的残基也导致对于较慢的总反应升-Arg; 但是,它们可以实现较高的底物选择性。我们的研究强调了第二配位球在蛋白质中的重要性,该蛋白质可定位底物和氧化剂,扰动电荷分布并实现底物选择性。
更新日期:2021-03-04
中文翻译:

蛋白质的静电扰动如何影响非血红素依赖性铁霉素丝裂霉素生物合成酶中底物羟化与去饱和的分叉途径?
紫霉素生物合成酶VioC是非血红素铁和α-酮戊二酸依赖性双加氧酶,参与抗生素生物合成的C 3位上1-精氨酸的选择性羟基化。有趣的是,实验研究表明,使用底物类似物,即1 -homo-精氨酸,获得了源自C 3-羟基化,C 4-羟基化和C 3 -C 4-去饱和的产物混合物。了解如何添加一个CH 2基团与底物的结合可能导致选择性和活性的急剧变化,我们决定使用量子力学(QM)簇模型进行计算研究。我们建立了一个包含245个原子的大型活性位点簇模型,其中包括氧化剂及其第一,第二配位球的影响以及底物的结合口袋。该模型已针对相关酶文献和先前的计算研究的实验工作进行了验证。此后,针对含有1 -Arg的模型和以1 -homo-Arg作为底物的模型研究了导致产物和副产物的可能途径。计算出的活化自由能预测与实验观察结果相符的产物分布,并给出低能的C 3。羟基化途径升-精氨酸,而升-homo精氨酸,一些障碍被发现是接近能源导致的产物的混合物。然后,我们使用热化学,价键和静电模型分析了产品分布差异的起因。我们的研究表明,l -Arg和l -homo-Arg的C 3 -H和C 4 -H键强度相似;然而,蛋白质感应电场的外部扰动会显着影响l -Arg的相对C–H键强度,并使C 3 –H键最弱,并将反应引导至选择性C 3-羟基化通道。因此,蛋白质的电荷分布和VioC活性位点的感应电偶极子场会引导1- Arg底物活化至C 3-羟基化,并不利于C 4-羟基化途径,而对于l -homo不会发生-Arg 紧基板定位和静电扰动从VIOC第二配位球的残基也导致对于较慢的总反应升-Arg; 但是,它们可以实现较高的底物选择性。我们的研究强调了第二配位球在蛋白质中的重要性,该蛋白质可定位底物和氧化剂,扰动电荷分布并实现底物选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号