当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revisiting bone morphogenetic protein‐2 knuckle epitope and redesigning the epitope‐derived peptides
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-02-22 , DOI: 10.1002/psc.3309 Guangzong Zhao 1 , Longqiang Zhang 1 , Lifan Che 2 , Huazhuang Li 1 , Yao Liu 2 , Jun Fang 1
Journal of Peptide Science ( IF 1.8 ) Pub Date : 2021-02-22 , DOI: 10.1002/psc.3309 Guangzong Zhao 1 , Longqiang Zhang 1 , Lifan Che 2 , Huazhuang Li 1 , Yao Liu 2 , Jun Fang 1
Affiliation
|
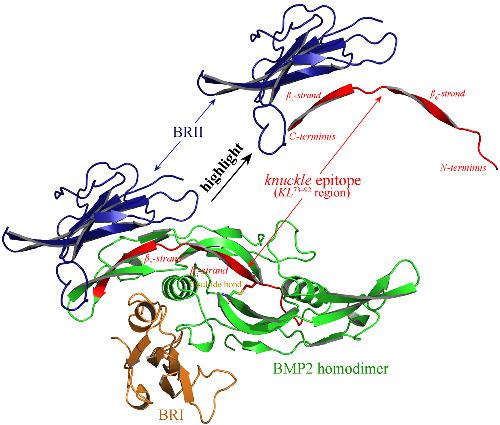
The bone morphogenetic protein‐2 (BMP2) plays a crucial role in bone formation, growth and regeneration, which adopts a conformational wrist epitope and a linear knuckle epitope to interact with its type‐I (BRI) and type‐II (BRII) receptors, respectively. In this study, we systematically examine the BRII‐recognition site of BMP2 at structural, energetic and dynamic levels and accurately locate hotspots of the recognition at BMP2–BRII complex interface. It is revealed that the traditional knuckle epitope (BMP2 residue range 73–92) do fully match the identified hotspots; the BMP2‐recognition site includes the C‐terminal region of traditional knuckle epitope as well as its flanked β‐strands. In addition, the protein context of full‐length BMP2 is also responsible for the recognition by addressing conformational constraint on the native epitope segment. Therefore, we herein redefine the knuckle epitope to BMP2 residue range 84–102, which has a similar sequence length but is slid along the protein sequence by ~10 residues as compared to traditional knuckle epitope. The redefined one is also a linear epitope that is natively a double‐stranded β‐sheet with two asymmetric arms as compared to the natively single β‐strand of the traditional version, although their sequences are partially overlapped to each other. It is revealed that the redefined epitope‐derived peptide LN84–102 exhibits an improved affinity by >3‐fold relative to the traditional epitope‐derived peptide KL73–92. Even so, the LN84–102 peptide still cannot fully represent the BMP2 recognition event by BRII that has been reported to have a nanomolar affinity. We further introduce a disulfide bond across the two arms of double‐stranded β‐sheet to constrain the free LN84–102 peptide conformation, which mimics the conformational constraint addressed by protein context. Consequently, several cyclic peptides are redesigned, in which the LN84–102(cyc89‐101) is determined to exhibit a sub‐micromolar affinity; this value is ~5‐fold higher than its linear counterpart. Structural analysis also reveals that the cyclic peptide can interact with BRII in a similar binding mode with the redefined knuckle epitope region in full‐length BMP2 protein.
中文翻译:

重新审视骨形态发生蛋白-2 关节表位并重新设计表位衍生肽
骨形态发生蛋白-2 (BMP2) 在骨形成、生长和再生中起着至关重要的作用,它采用构象腕部表位和线性关节表位与其 I 型 (BRI) 和 II 型 (BRII) 受体相互作用, 分别。在这项研究中,我们系统地检查了 BMP2 在结构、能量和动态水平上的 BRII 识别位点,并准确定位了 BMP2-BRII 复合界面的识别热点。结果表明,传统的指关节表位(BMP2 残基范围 73-92)与识别的热点完全匹配;BMP2 识别位点包括传统关节表位的 C 端区域及其两侧的β-股。此外,全长 BMP2 的蛋白质上下文也负责通过解决天然表位片段的构象限制来识别。因此,我们在此将关节表位重新定义为 BMP2 残基范围 84-102,其具有相似的序列长度,但与传统的关节表位相比,它沿蛋白质序列滑动了约 10 个残基。重新定义的表位也是一个线性表位,与传统版本的天然单β链相比,它本身是具有两个不对称臂的双链β折叠,尽管它们的序列彼此部分重叠。结果表明,重新定义的表位衍生肽LN 84-102与传统的表位衍生肽KL 73-92相比,亲和力提高了 3 倍以上。即便如此,LN 84-102肽仍然不能完全代表 BRII 的 BMP2 识别事件,据报道它具有纳摩尔亲和力。我们进一步在双链β折叠的两个臂上引入二硫键以限制游离的LN 84-102肽构象,这模拟了蛋白质上下文解决的构象约束。因此,重新设计了几种环肽,其中LN 84-102(cyc89-101) 被确定表现出亚微摩尔亲和力;该值比其线性对应值高约 5 倍。结构分析还表明,环肽可以与全长 BMP2 蛋白中重新定义的关节表位区域类似的结合模式与 BRII 相互作用。
更新日期:2021-04-28
中文翻译:

重新审视骨形态发生蛋白-2 关节表位并重新设计表位衍生肽
骨形态发生蛋白-2 (BMP2) 在骨形成、生长和再生中起着至关重要的作用,它采用构象腕部表位和线性关节表位与其 I 型 (BRI) 和 II 型 (BRII) 受体相互作用, 分别。在这项研究中,我们系统地检查了 BMP2 在结构、能量和动态水平上的 BRII 识别位点,并准确定位了 BMP2-BRII 复合界面的识别热点。结果表明,传统的指关节表位(BMP2 残基范围 73-92)与识别的热点完全匹配;BMP2 识别位点包括传统关节表位的 C 端区域及其两侧的β-股。此外,全长 BMP2 的蛋白质上下文也负责通过解决天然表位片段的构象限制来识别。因此,我们在此将关节表位重新定义为 BMP2 残基范围 84-102,其具有相似的序列长度,但与传统的关节表位相比,它沿蛋白质序列滑动了约 10 个残基。重新定义的表位也是一个线性表位,与传统版本的天然单β链相比,它本身是具有两个不对称臂的双链β折叠,尽管它们的序列彼此部分重叠。结果表明,重新定义的表位衍生肽LN 84-102与传统的表位衍生肽KL 73-92相比,亲和力提高了 3 倍以上。即便如此,LN 84-102肽仍然不能完全代表 BRII 的 BMP2 识别事件,据报道它具有纳摩尔亲和力。我们进一步在双链β折叠的两个臂上引入二硫键以限制游离的LN 84-102肽构象,这模拟了蛋白质上下文解决的构象约束。因此,重新设计了几种环肽,其中LN 84-102(cyc89-101) 被确定表现出亚微摩尔亲和力;该值比其线性对应值高约 5 倍。结构分析还表明,环肽可以与全长 BMP2 蛋白中重新定义的关节表位区域类似的结合模式与 BRII 相互作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号