Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-02-23 , DOI: 10.1016/j.jhazmat.2021.125483 Zibo Xu , Xiaoyun Xu , Yulu Yu , Chengbo Yao , Daniel C.W. Tsang , Xinde Cao
|
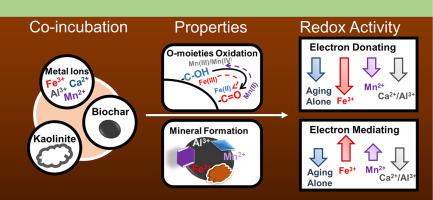
Biochar in soil is susceptible to natural aging along with soil minerals, which might alter its electrochemical properties and redox reactions with contaminants. In this study, soluble mineral salts (FeCl3, MnCl2, AlCl3, CaCl2) and clay mineral (kaolinite) were selected to investigate the impact of co-aging with soil minerals on the redox activity of peanut-shell biochar for Cr(VI) reduction. Natural aging for 3-month induced oxidation of biochar with the decrease of reducing moieties, i.e., ‒C‒OH from 26.8–43.7% to 18.4–24.1%. Co-aging with minerals except for Mn(II) further decreased the proportion of ‒C‒OH to 6.94–22.2% because of the interaction between mineral ions and biochar, resulting in the formation of mineral-biochar complex and new minerals, e.g. β-FeOOH. Due to its reductivity, Mn(II) presented the least decrease or even slight increase of ‒C‒OH while itself was oxidized to Mn(III) and Mn(IV). The decline of ‒C‒OH caused the decrease of Cr(VI) reduction rate constant from 2.18 to 2.47 × 10-2 h-1 for original biochars to 0.71–1.95 × 10−2 h−1 for aged ones, of which co-aging with Fe(III) showed the lowest reduction rate constant among all minerals. The electron mediating capacity of biochar also decreased after aging alone or co-aging with Al, Ca, and kaolinite, while co-aging with Fe(III) and Mn(II) facilitated the electron transfer process, increasing the rate constant by 219.3–1237% due to electron mediation through valence transformation of Fe(III)-Fe(II) and Mn(II)-Mn(III). Given the abundance of soil minerals, it was essential to consider this crucial factor for redox reactions when applying biochar for soil remediation.
中文翻译:

与土壤矿物相互作用过程中生物炭氧化还原活性的演变:对Cr(VI)还原的电子给体和介电能力的影响
土壤中的生物炭易与土壤矿物质一起自然老化,这可能会改变其电化学性质以及与污染物的氧化还原反应。在这项研究中,可溶性矿物盐(FeCl 3,MnCl 2,AlCl 3,CaCl 2)和粘土矿物(高岭石)被选择来研究与土壤矿物共同老化对花生壳生物炭还原Cr(VI)的氧化还原活性的影响。3个月的自然老化导致生物炭氧化,还原部分减少,即‒C‒OH从26.8–43.7%减少到18.4–24.1%。由于矿物离子与生物炭之间的相互作用,与锰(II)以外的矿物共同时效进一步将‒C‒OH的比例降低至6.94-2.2%,从而形成了矿物-生物炭复合物和新的矿物,例如β -FeOOH。由于其还原性,Mn(II)的‒C‒OH减少最少,甚至略有增加,而其本身被氧化为Mn(III)和Mn(IV)。‒C‒OH的下降导致Cr(VI)还原速率常数从2.18下降到2.47×10 -2 h -1原始生物炭的老化速率为0.71-1.95×10 -2 h -1,其中与Fe(III)共同老化显示所有矿物中还原速率常数最低。单独老化或与Al,Ca和高岭石共同老化后,生物炭的电子介电能力也降低,而与Fe(III)和Mn(II)共同老化促进了电子转移过程,将速率常数提高了219.3–由于电子通过Fe(III)-Fe(II)和Mn(II)-Mn(III)的化合价进行介导,因此达到1237%。考虑到土壤矿物质的丰富性,在应用生物炭进行土壤修复时,必须考虑到氧化还原反应的这一关键因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号