Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2021-02-23 , DOI: 10.1016/j.jcou.2021.101476 Azeem Sarwar , Majid Ali , Asif Hussain Khoja , Azra Nawar , Adeel Waqas , Rabia Liaquat , Salman Raza Naqvi , Muhammad Asjid
|
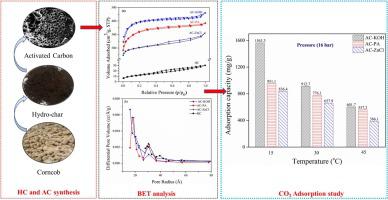
In this study, activated carbon (AC) based adsorbents were synthesized from biomass-derived hydro-char (HC) through modified hydrothermal carbonization (HTC) process coupled with H3PO4 (PA), ZnCl2, (ZnCl), and KOH thermochemical activation with a specific impregnation ratio of 1:3 (HC/activating agent) at 600 °C. The prepared ACs were characterized through CHN-S analyser, XRD, SEM/EDS, BET, TGA, and FTIR analysis. The modification in textural and surface morphology has been observed. Thermochemical activation results in surface modification of AC samples with a higher specific (SBET) surface area having a range of 650−1225 m2/g, large microporous volume (VμD-A, VμD–R) up to 0.624 and 0.642 cm3/g with 68–90 % micro-porosity. The CO2 adsorption capacity was examined through a high-pressure gas sorption analyser at a pressure of 0−16 bar and temperature of 15 °C, 30 °C, and 45 °C. At 15 °C of temperature, AC-PA showed the adsorption capacity of 130 and 958 mg/g, while AC-ZnCl exhibited 160 and 836 mg/g of adsorption at 1 and 16 bar of pressure respectively. Whereas AC-KOH exhibited a notable CO2 adsorption capacity of 198 and 1560 mg/g at 1 and 16 bar of pressure (15 °C). Furthermore, experimental equilibrium data of CO2 adsorption were analysed by applying Freundlich, Langmuir, Redlich-Peterson, Sips, and Toth isotherm models and validate these models by calculating the regression coefficient (R2), and standard deviation Δq (%). Finally, the thermodynamics parameters (ΔH°, ΔS°, ΔG°, and ΔHIsosteric) were evaluated and concluded that the adsorption of CO2 (adsorbate) on adsorbent is spontaneous and exothermic.
中文翻译:

增强CO 2吸附的生物质衍生的表面改性活性炭的合成与表征
在这项研究中,活性炭(AC)为基础的吸附剂是由生物质衍生的碳氢化合物(HC)通过改进的水热碳化(HTC)工艺与H 3 PO 4(PA),ZnCl 2(ZnCl)和KOH合成的在600°C下以1:3的特定浸渍比(HC /活化剂)进行热化学活化。通过CHN-S分析仪,XRD,SEM / EDS,BET,TGA和FTIR分析对制备的AC进行表征。已经观察到纹理和表面形态的改变。热化学活化导致具有较高比表面积(S BET)的AC样品的表面改性,比表面积(S BET)范围为650-1225 m 2 / g,微孔体积较大(VμD-A,V μD–R)高达0.624和0.642 cm 3 / g,微孔率为68–90%。通过高压气体吸附分析仪在0-16 bar的压力和15°C,30°C和45°C的温度下检查了CO 2的吸附能力。在15°C的温度下,AC-PA的吸附容量为130和958 mg / g,而AC-ZnCl在1 bar和16 bar的压力下分别显示160和836 mg / g的吸附量。而AC-KOH在1和16 bar的压力(15°C)下表现出明显的198和1560 mg / g的CO 2吸附容量。此外,通过使用Freundlich,Langmuir,Redlich-Peterson,Sips和Toth等温线模型分析了CO 2吸附的实验平衡数据,并通过计算回归系数(R2)和标准偏差Δq(%)。最后,评估了热力学参数(ΔH°,ΔS°,ΔG°和ΔHIsosteric),并得出结论,吸附剂对CO 2(被吸附物)的吸附是自发的且放热的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号