Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-02-23 , DOI: 10.1016/j.bmc.2021.116085 Maurício Temotheo Tavares 1 , Larissa Costa de Almeida 2 , Thales Kronenberger 3 , Glaucio Monteiro Ferreira 4 , Thainá Fujii de Divitiis 1 , Mônica Franco Zannini Junqueira Toledo 1 , Neuza Mariko Aymoto Hassimotto 5 , João Agostinho Machado-Neto 2 , Letícia Veras Costa-Lotufo 2 , Roberto Parise-Filho 1
|
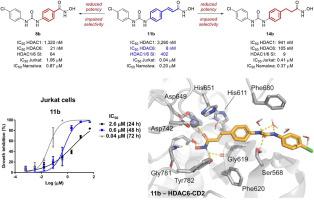
Histone deacetylases (HDACs) are a family of enzymes that modulate the acetylation status histones and non-histone proteins. Histone deacetylase inhibitors (HDACis) have emerged as an alternative therapeutic approach for the treatment of several malignancies. Herein, a series of urea-based cinnamyl hydroxamate derivatives is presented as potential anticancer HDACis. In addition, structure-activity relationship (SAR) studies have been performed in order to verify the influence of the linker on the biological profile of the compounds. All tested compounds demonstrated significant antiproliferative effects against solid and hematological human tumor cell lines. Among them, 11b exhibited nanomolar potency against hematological tumor cells including Jurkat and Namalwa, with IC50 values of 40 and 200 nM, respectively. Cellular and molecular proliferation studies, in presence of compounds 11a-d showed significant cell growth arrest, apoptosis induction, and up to 43-fold selective cytotoxicity for leukemia cells versus non-tumorigenic cells. Moreover, compounds 11a-d increased acetylated α-tubulin expression levels, which is phenotypically consistent with HDAC inhibition, and indirectly induced DNA damage. In vitro enzymatic assays performed for 11b revealed a potent HDAC6 inhibitory activity (IC50: 8.1 nM) and 402-fold selectivity over HDAC1. Regarding SAR analysis, the distance between the hydroxamate moiety and the aromatic ring as well as the presence of the double bond in the cinnamyl linker were the most relevant chemical feature for the antiproliferative activity of the series. Molecular modeling studies suggest that cinnamyl hydroxamate is the best moiety of the series for binding HDAC6 catalytic pocket whereas exploration of Ser568 by the urea connecting unity (CU) might be related with the selectivity observed for the cinnamyl derivatives. In summary, cinnamyl hydroxamate derived compounds with HDAC6 inhibitory activity exhibited cell growth arrest and increased apoptosis, as well as selectivity to acute lymphoblastic leukemia cells. This study explores interesting compounds to fight against neoplastic hematological cells.
中文翻译:

一系列异羟肟酸肉桂酯组蛋白脱乙酰酶抑制剂的构效关系及机理研究
组蛋白去乙酰化酶 (HDAC) 是一个酶家族,可调节组蛋白和非组蛋白的乙酰化状态。组蛋白去乙酰化酶抑制剂 (HDACis) 已成为治疗多种恶性肿瘤的替代治疗方法。在此,一系列基于尿素的异羟肟酸肉桂酯衍生物被提出作为潜在的抗癌 HDACis。此外,还进行了构效关系 (SAR) 研究以验证接头对化合物生物学特性的影响。所有测试的化合物都显示出对实体和血液人类肿瘤细胞系的显着抗增殖作用。其中,11b对包括 Jurkat 和 Namalwa 在内的血液肿瘤细胞表现出纳摩尔级效力,IC 50值分别为 40 和 200 nM。细胞和分子增殖研究,在化合物11a-d 的存在下显示出显着的细胞生长停滞、细胞凋亡诱导和高达 43 倍的白血病细胞与非致瘤细胞的选择性细胞毒性。此外,化合物11a-d增加了乙酰化 α-微管蛋白的表达水平,这与 HDAC 抑制的表型一致,并间接诱导了 DNA 损伤。对11b进行的体外酶促测定显示出有效的 HDAC6 抑制活性(IC 50: 8.1 nM) 和比 HDAC1 高 402 倍的选择性。关于 SAR 分析,异羟肟酸酯部分与芳环之间的距离以及肉桂基接头中双键的存在是与该系列抗增殖活性最相关的化学特征。分子建模研究表明,肉桂基异羟肟酸酯是该系列中结合 HDAC6 催化口袋的最佳部分,而通过尿素连接单位 (CU) 对 Ser568 的探索可能与观察到的肉桂基衍生物的选择性有关。总之,具有 HDAC6 抑制活性的肉桂基异羟肟酸酯衍生化合物表现出细胞生长停滞和细胞凋亡增加,以及对急性淋巴细胞白血病细胞的选择性。这项研究探索了有趣的化合物来对抗肿瘤性血液细胞。









































 京公网安备 11010802027423号
京公网安备 11010802027423号