Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2021-02-22 , DOI: 10.1016/j.saa.2021.119613 Bahia Abbas Moussa , Hanaa M.A. Hashem , Marianne Alphonse Mahrouse , Sally Tarek Mahmoud
|
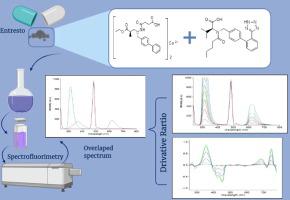
A sensitive and accurate spectrofluorimetric method was proposed for the determination of Sacubitril calcium and Valsartan simultaneously in binary mixture. The method was established on measuring the native fluorescence of Sacubitril calcium and Valsartan upon excitation at 240 nm in acetonitrile. The emission of Sacubitril calcium was measured at 615 nm. For the determination Valsartan a first derivative ratio method was employed to eliminate any spectral interference. The ratio emission spectra were achieved by dividing the emission spectra of various concentrations of Valsartan by the emission spectrum of Sacubitril calcium (100 ng/ml) then the first derivative of the obtained ratio emission spectra was recorded using the proper smoothing factor. The amplitude at 354.9 nm on the first derivative ratio emission spectrum was used to calculate the concentrations of Valsartan in presence of Sacubitril calcium. The method was linear over the concentration range 100–1000 ng/ml for both Sacubitril calcium and Valsartan. The mean accuracy values were found to be 99.32 ± 0.62 and 99.30 ± 0.70 for Sacubitril calcium and Valsartan, respectively. Statistical comparison between results obtained by the proposed method and a reported method for this drugs showed no significant difference. This developed method was used for the quantitative determination of Sacubitril calcium and Valsartan in both pure and pharmaceutical dosage form.
中文翻译:

经验证的荧光分光光度法同步测定含沙比特利钙和缬沙坦的新型心力衰竭联合疗法
提出了一种灵敏而准确的荧光分光光度法同时测定二元混合物中的cu草酸钙和缬沙坦。建立了在乙腈中于240 nm激发下测量沙必比尔钙和缬沙坦的天然荧光的方法。在615 nm处测量了Sacubitril钙的发射。为了测定缬沙坦,采用一阶导数比方法消除了任何光谱干扰。通过将各种浓度的缬沙坦的发射光谱除以沙必比尔钙(100 ng / ml)的发射光谱来获得比率发射光谱,然后使用适当的平滑因子记录所获得的比率发射光谱的一阶导数。354处的振幅。使用一阶导数比发射光谱上的9 nm来计算在存在屈比特尔钙的情况下缬沙坦的浓度。该方法在Sacubitril钙和Valsartan的浓度范围为100–1000 ng / ml时都是线性的。沙必比尔钙和缬沙坦的平均准确度值分别为99.32±0.62和99.30±0.70。通过该药物的拟议方法与报道的方法获得的结果之间的统计比较显示无显着差异。这种发达的方法可用于定量测定纯制剂和药物剂型中的杀必特尔钙和缬沙坦。沙必比尔钙和缬沙坦的平均准确度值分别为99.32±0.62和99.30±0.70。通过该药物的拟议方法与报道的方法获得的结果之间的统计比较显示无显着差异。这种发达的方法可用于定量测定纯制剂和药物剂型中的杀必特尔钙和缬沙坦。沙必比尔钙和缬沙坦的平均准确度值分别为99.32±0.62和99.30±0.70。通过该药物的拟议方法与报道的方法获得的结果之间的统计比较显示无显着差异。这种发达的方法可用于定量测定纯制剂和药物剂型中的杀必特尔钙和缬沙坦。











































 京公网安备 11010802027423号
京公网安备 11010802027423号