Chemosphere ( IF 8.1 ) Pub Date : 2021-02-22 , DOI: 10.1016/j.chemosphere.2021.130057 Yang-wen Wu , Xin-yue Zhou , Teng-ge Mi , Zhuang Hu , Ming-xin Xu , Bing Zhang , Li Zhao , Qiang Lu
|
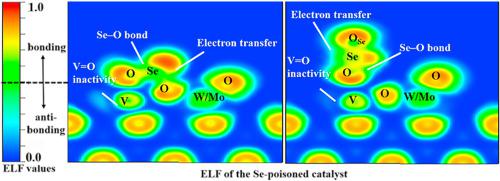
Selenium (Se) species can deposit in selective catalytic reduction (SCR) system during the denitrification process, which is harmful to the catalyst. To improve the Se-poisoning resistance of SCR catalysts, the influence mechanism of Se species on vanadium-titanium-based catalysts should be elucidated from an atomic scale. In this paper, theoretical calculations were conducted to reveal the adsorption and interaction mechanism of Se species on V2O5-WO3(MoO3)/TiO2 surface based on the first-principles. The impact of Se species on the electronic structure of the catalyst was investigated from electron transfer, bond formation, and V O site activity. The results show that the adsorption of elementary Se (Se0) belongs to chemisorption, while SeO2 can undergo both physisorption and chemisorption. For the chemisorption of Se species, obvious charge transfer with the catalyst is observed and Se–O bond is formed, which enhances the oxidation activity of the catalyst, triggers the reaction of Se0 and SeO2 with the catalyst components to generate SeVOx and SeW(Mo)Ox. The active sites are thereby damaged and the SCR performance is reduced. The above conclusions are mutually confirmed with the previous experimental research. By studying the correlation with the adsorption energies of Se species, descriptors manifesting the Se species adsorption were initially investigated to unveil the relationship between the electronic structure and the adsorption energy. Finally, the influence of temperature on Se adsorption was investigated. The adsorption can only proceed spontaneously below 500 K and is inhibited at high temperatures.
O site activity. The results show that the adsorption of elementary Se (Se0) belongs to chemisorption, while SeO2 can undergo both physisorption and chemisorption. For the chemisorption of Se species, obvious charge transfer with the catalyst is observed and Se–O bond is formed, which enhances the oxidation activity of the catalyst, triggers the reaction of Se0 and SeO2 with the catalyst components to generate SeVOx and SeW(Mo)Ox. The active sites are thereby damaged and the SCR performance is reduced. The above conclusions are mutually confirmed with the previous experimental research. By studying the correlation with the adsorption energies of Se species, descriptors manifesting the Se species adsorption were initially investigated to unveil the relationship between the electronic structure and the adsorption energy. Finally, the influence of temperature on Se adsorption was investigated. The adsorption can only proceed spontaneously below 500 K and is inhibited at high temperatures.
中文翻译:

原理对选择性催化还原催化剂上硒的吸附和相互作用机理的见解
硒(Se)物质在反硝化过程中会沉积在选择性催化还原(SCR)系统中,这对催化剂有害。为了提高SCR催化剂的抗Se中毒性,应从原子尺度上阐明Se物种对钒钛基催化剂的影响机理。本文基于第一性原理进行了理论计算,揭示了Se在V 2 O 5 -WO 3(MoO 3)/ TiO 2表面上的吸附和相互作用机理。通过电子转移,键形成和钒研究了硒物种对催化剂电子结构的影响。 O站点活动。结果表明,元素Se(Se 0)的吸附属于化学吸附,而SeO 2既可以进行物理吸附也可以进行化学吸附。对于Se物种的化学吸附,观察到了明显的与催化剂的电荷转移,并形成了Se–O键,这增强了催化剂的氧化活性,触发了Se 0和SeO 2与催化剂成分的反应,生成SeVO x和硒(Mo)O x。从而损坏了活性部位并且降低了SCR性能。以上结论与以往的实验研究相互证实。通过研究与硒物质吸附能的相关性,初步研究了表征硒物质吸附的描述子,揭示了电子结构与吸附能之间的关系。最后,研究了温度对硒吸附的影响。吸附只能在低于500 K的条件下自发进行,并且在高温下会被抑制。
O站点活动。结果表明,元素Se(Se 0)的吸附属于化学吸附,而SeO 2既可以进行物理吸附也可以进行化学吸附。对于Se物种的化学吸附,观察到了明显的与催化剂的电荷转移,并形成了Se–O键,这增强了催化剂的氧化活性,触发了Se 0和SeO 2与催化剂成分的反应,生成SeVO x和硒(Mo)O x。从而损坏了活性部位并且降低了SCR性能。以上结论与以往的实验研究相互证实。通过研究与硒物质吸附能的相关性,初步研究了表征硒物质吸附的描述子,揭示了电子结构与吸附能之间的关系。最后,研究了温度对硒吸附的影响。吸附只能在低于500 K的条件下自发进行,并且在高温下会被抑制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号